Medicaid Coverage of Family Planning Benefits: Results from a State Survey
Cervical and Breast Cancer Services
The survey asked states about their policies with respect to coverage of services for cervical and breast cancers under their traditional programs and family planning expansion programs. These include the HPV vaccine, pap smear and follow up testing after abnormal laboratory results, mammograms, genetic (BRCA) screening for high-risk women, and breast cancer preventive medication for high-risk women. These services are required coverage for the ACA expansion group as they are recommended by the US Preventive Services Task Force.
Cervical Cancer Services – PAP Test and Follow Up, HPV Vaccine
| Key Finding: Cervical Cancer Services |
| All states cover Pap screening for cervical cancer regardless of eligibility pathway, but follow-up tests for abnormal screening results are less likely to be covered in state family planning waivers or SPAs. Of the states that do provide coverage, many indicate that the procedures or services are covered as part of a family planning visit under a family planning waiver or SPA, rather than as a specific benefit. |
The survey inquired about coverage of pap and lab testing as well as additional screening procedures subsequent to an abnormal result from the pap test. These procedures include:
- Colposcopy is a procedure to examine the cervix following abnormal pap smear results. The procedure may include extracting a small sample of tissue (biopsy) during the examination.
- LEEP, Loop Electrosurgical Excision Procedure, is a treatment for abnormal cells on the cervix, using a thin wire loop that has an electric current to remove the abnormal tissue. LEEP may be performed after abnormal cells are discovered during a pap test, colposcopy or biopsy.
- HPV DNA testing consists of using one of various biologic tests on a sample of tissue to detect the presence of DNA or RNA from the human papilloma virus.
Coverage across Eligibility Pathways
All of the states responding to the survey provide coverage for pap smear and lab in all three Medicaid pathways. All states participating in the survey cover the three follow-up screening methods in their traditional Medicaid and ACA Medicaid expansion programs, except North Carolina, which does not cover HPV DNA testing through any Medicaid pathway offered in the state. Coverage of the three follow-up screening methods varies more in state family planning programs (Table 16). Five states do not cover any of the three follow-up procedures in their family planning waiver or SPA: North Carolina, Oklahoma, Oregon, Virginia and Washington. Four states cover HPV DNA testing within their family planning waiver or SPA, but do not cover colposcopy or LEEP: Alabama, Michigan, South Carolina and Wyoming. Missouri covers colposcopy and HPV DNA testing, but does not cover LEEP.
All but one state (South Carolina) cover HPV vaccines for young adults in their traditional Medicaid programs, but only 14 of 23 states cover HPV vaccines in their family planning waiver or SPA despite the widespread coverage for pap screening. States must cover the vaccine for ACA expansion groups, as all vaccines recommended by the national Advisory Committee on Immunization Practices (ACIP) are included in the ACA’s preventive services coverage requirement.
Utilization Controls
Only two states noted utilization controls for cancer screening methods. California restricts utilization to women ages 21 through 65 regardless of sexual history. Colorado limits a pap smear and lab to one per year unless additional screens are determined to be medically necessary.
| Table 15: Coverage of Cervical Cancer Services | ||||
| Traditional Medicaid (n=41) |
ACA Medicaid Expansion (n=25) |
Family Planning Waiver/SPA (n=23) |
Covered in Context of Family Planning Visit | |
| HPV Vaccine | 40† | Required | 14: AL, CT, IA, IN, MD, MN, MO, MS, MT, NH, NM, OH, OK, WY | |
| PAP Smear and Lab | 41 | Required | 23: AL, CA, CT, GA, IA, IN, MD, MI, MN, MO, MS, MT, NC, NH, NM, NY, OH, OK, OR, SC, VA, WA, WY | CA, IA*, MO, OR*, VA |
| Follow- up Procedures with Abnormal Pap | ||||
| Colposcopy | 41 | 24 | 14: CA, CT, GA, IA, IN, MD, MN, MO, MS, MT, NH, NM, NY, OH | IA*, MO |
| LEEP | 41 | 24 | 13: CA, CT, GA, IA, IN, MD, MN, MS, MT, NH, NM, NY, OH | |
| HPV DNA Testing | 40ǂ | Required | 18: AL, CA, CT, GA, IA, IN, MD, MI, MN, MO, MS, MT, NH, NM, NY, OH, SC, WY | MO |
| Note: † Not covered in SC. ǂ Not covered in NC. *IA: sometimes considered family planning, depending on context of the visit. OR: Pap covered under state family planning waiver when provided in context of a contraceptive management visit. | ||||
Breast Cancer Services
| Key Finding: Breast Cancer Services |
| Breast cancer screening is a required benefit in ACA Medicaid expansion programs, but it is considered “optional” under traditional Medicaid; however, all states covered this screening under these two programs.
Few states cover breast cancer screening and prevention services through their family planning waiver or SPA. |
The USPSTF recommends three services for women related to detection and prevention of breast cancer: 1) biannual mammograms for women ages 50 to 74 to screen for breast cancer; 2) genetic testing for mutation of the BRCA1 and BRCA2 genes in some women with family members that have breast, ovarian, fallopian tube, or peritoneal cancer; 3) preventive medication for some women with elevated risk of breast cancer. Because these services are all recommended by the USPSTF, they must be covered in Medicaid ACA expansion programs but are not required in traditional or family planning pathways.
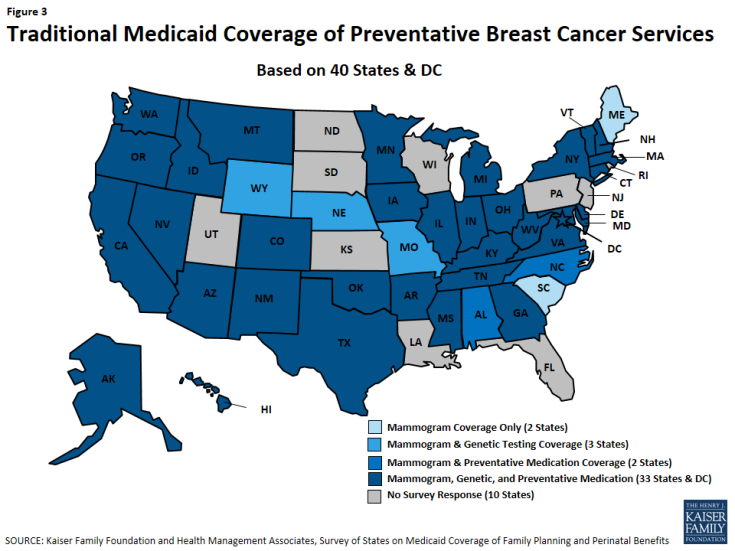
Minnesota, Montana, New Hampshire, and New Mexico cover all three services across all of their eligibility pathways. Thirty-three states and DC cover all of these services under traditional Medicaid (Figure 3). All states cover mammograms, and most cover genetic BRCA screening (37 of 41 states), and breast cancer preventive medication (36 of 41 states) for high-risk women under their traditional Medicaid program (Table 17). Maine and South Carolina do not cover BRCA Screening/Counseling nor breast cancer preventive medication in any of the pathways where coverage for these services is optional.
Coverage of breast cancer screening and prevention under state family planning waivers or SPAs is much less common, with seven of 23 of these programs covering mammograms (Maryland, Minnesota, Montana, New Hampshire, and New Mexico, Ohio, South Carolina) and six states covering BRCA screening and counseling (Minnesota, Montana, New Hampshire, and New Mexico Maryland, Iowa) or preventive breast cancer medication (Minnesota, Montana, New Hampshire, and New Mexico, Ohio, Iowa).
Utilization Controls
No states noted utilization controls for mammograms. Five states have utilization controls applied to BRCA screening and counseling. Michigan, Texas, Vermont and Washington require prior authorization and in Nevada, genetic counseling must precede the testing. Michigan requires prior authorization for preventive medications. Appendix Table A9 provides detail for state responses on breast cancer services.
| Table 17: Coverage for Breast Cancer Screening and Prevention | |||
| Traditional Medicaid (n=41) |
ACA Medicaid Expansion (n=25) |
Family Planning Waiver/SPA (n=23) |
|
| Mammogram | 41 | Required | 7 |
| Genetic (BRCA) Screening and Counseling for High-Risk Women | 37 | Required | 6 |
| Breast Cancer Preventive Medication for High-Risk Women | 36 | Required | 6 |