Medication Abortion and Telemedicine: Innovations and Barriers During the COVID-19 Emergency
Amrutha Ramaswamy, Gabriela Weigel, Laurie Sobel, and Alina Salganicoff
Published:
| UPDATE |
|
On April 12th, 2021, the FDA’s Center for Drug Evaluation and Research (CDER) notified ACOG that they will “exercise enforcement discretion” during the ongoing public health emergency with respect to in-person dispensing requirements of mifepristone based on a safety review. Effectively, this will allow providers in states that do not have laws that would otherwise ban this practice to dispense mifepristone using the telehealth protocol for medication abortion. On May 7th, 2021, in response to the ACLU lawsuit, the FDA announced in a court filing that a review of the REMS is currently underway.
|
State actions in response to the COVID-19 crisis have highlighted their divergent approaches to abortion access. Some states classified abortion as a non-essential service, effectively banning services, while others have clarified that abortion is an essential service. In a handful of states, some clinics have begun to offer medication abortions using telemedicine. This approach maintains access to abortion while social distancing, preserving personal protective equipment (PPE), and limiting in-person health care visits and risk of exposure.
In 2017, 39% of all abortions in the U.S. were medication abortions (also known as abortions induced by pills). These abortions are provided using two medications, mifepristone and misoprostol. While public knowledge about medication abortion is very low, even fewer people may be aware that telemedicine can aid in the provision of this service. Research shows that providing medication abortion by telemedicine is clinically feasible and safe, but COVID-19 has highlighted the impact of new and existing federal and state restrictions on providing abortions using this approach.
The Telehealth for Medication Abortion Protocol
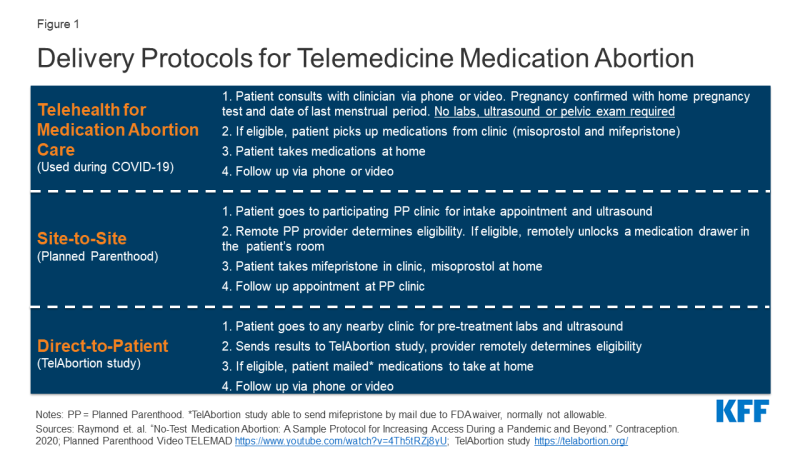
In an effort to preserve abortion access and maintain patient and provider safety during the COVID-19 pandemic, reproductive health care researchers and providers from Gynuity Health Projects, Advancing New Standards in Reproductive Health (ANSIRH) at UCSF, and the Society of Family Planning, among others, developed a “Telehealth for Medication Abortion Care” protocol for providing medication abortion building on prior research. This method enables patients to obtain their abortions safely without the traditional need for in-person pregnancy testing, pelvic examination, ultrasound or labs. Clinicians are able to evaluate patients remotely via a telehealth visit (e.g. video or phone) to determine their clinical eligibility for this service based on their health and gestational age. If eligible, patients pick up their abortion medications curbside from the clinic and take these pills after they get home. The follow-up visit with a clinician also happens via telehealth or through a phone call (Figure 1). Through this protocol, clinicians can provide abortion services while protecting patients and providers from SARS-CoV2 exposure. Maine Family Planning has implemented a similar protocol to eliminate the need for in-person testing.
International data support that telehealth for medication abortion protocols are safe and effective. In the UK, the Royal College of Obstetricians and Gynecologists recently approved a telehealth for medication abortion care protocol, with mifepristone available through a minimal-contact pick-up or by mail.
While this protocol is an innovative response to the provision of abortion services during the COVID-19 pandemic, using telemedicine to provide medication abortions is not new. In certain states, telemedicine abortions have been available through Planned Parenthood using a “site to site” protocol since 2008, and through the Gynuity Health Projects TelAbortion clinical trial using a “direct to patient” protocol since 2016. Gynuity is working to expand the number of states served by the TelAbortion study in response to the COVID-19 pandemic. This protocol, however, still necessitates some in-person care (e.g. ultrasound, blood tests). The protocol goes a step further in making medication abortions accessible during the COVID-19 emergency.
What Barriers Exist to Telemedicine Medication Abortions During the COVID-19 Emergency?
Only patients in a limited number of states had access to medication abortions via telehealth prior to the COVID-19 emergency. During the COVID-19 crisis, 12 states issued policies that attempted to limit abortion access during the outbreak, such as deeming abortion “non-essential.” Most of these state policies have been blocked by court order or lifted as states start to re-open. In Arkansas, patients were required to have had at least one negative COVID-19 Nucleic Acid Amplification Test (NAAT) test in the 48 hours prior to the procedure. Effective August 1st, the Arkansas Department of Health released another directive rescinding the requirement for a negative COVID-19 NAAT test prior to elective procedures.
These new restrictions are in addition to existing barriers to abortion services. In 19 states, telemedicine abortion has been effectively prohibited; 5 states explicitly ban telemedicine for medication abortion, while 14 states require the prescribing clinician be physically present with the patient. The telehealth protocol is also not an option in the 26 states requiring patients receive an ultrasound before an abortion, and in the 12 states with in-person counseling requirements. This leaves 22 states and DC in which the telehealth protocol could be used to provide medication abortion.
–
Another notable barrier to medication abortion is the Risk Evaluation and Mitigation Strategy (REMS) placed on mifepristone by the FDA, which only permits medical providers who have received special certification from the manufacturer to prescribe and dispense the drug. This requirement not only limits the number of clinicians able to prescribe medication abortions, but also means patients cannot obtain the medication from a retail pharmacy or by mail.
There have been calls to remove or relax the REMS requirement on mifepristone, but there has not yet been a response from the Trump Administration. Former head of the FDA, Jane Henney, has repeatedly called on the FDA to lift the REMS requirement. In March 2020, a coalition of 21 state attorneys general wrote to the FDA and the Department of Health and Human Services (HHS) asking them to waive mifepristone’s REMS to alleviate access issues related to in-person care. In April 2020, Senators Warren, Murray and Baldwin urged the FDA to “temporarily exercise enforcement discretion on in-person dispensing requirements,” so that people can access abortion services without risk of SARS-CoV-2 exposure.
In May 2020, the ACLU filed a lawsuit challenging the REMS requirement that mifepristone be dispensed in-person; this was filed on behalf of the American College of Obstetricians and Gynecologists (ACOG), in addition to other organizations. The United States District Court of Maryland ruled in favor of ACOG, preventing the FDA from enforcing the REMS for mifepristone, abortion medication during the COVID-19 pandemic. In August 2020, the FDA petitioned the requested that the Supreme Court for an emergency stay to lift the national injunction preventing the FDA from enforcing the REMS, contending that it is constitutional to impose a regulatory requirement on one method of abortion, even if it creates an undue burden on people seeking this method of abortion when another method is safe. On October 8, 2020, six weeks after the FDA’s request, to obtain a more comprehensive record, the Supreme Court issued an order suspending the case and directing the FDA to request the District Court to lift or modify the preliminary injunction. Justices Alito and Thomas dissented from the Court’s order. This unusual order to not rule on the stay until the FDA requests the District Court to reconsider the scope of the injunction may reflect a compromise because there were only eight justices when the Court issued the suspension.
On January 12, 2021, in a 6-3 ruling, the Supreme Court decided in favor of the Trump Administration, blocking the lower court’s ruling which had suspended the FDA REMS in–person dispensing requirements for mifepristone during the COVID-19 emergency. Justice Sotomayor wrote a dissent in which Justice Kagan joined, noting the FDA has made other accommodations in the cases of other medications to reduce the risk of exposure to SARS-Cov2. While the case continues to be litigation, abortion providers may not dispense mifepristone by mail to reduce the risk of exposure to COVID-19. However, this ruling does not prevent the FDA, under the Biden Administration, from suspending the REMS in–person dispensing requirements during the pandemic and thereby making the litigation moot. This ruling, until the FDA announcement in April 2021, prohibited providers from continuing to safely dispense mifepristone without the risk of exposure to the SARS-Cov2 as they have been doing since July 2020.
On April 12th, 2021, the FDA’s Center for Drug Evaluation and Research (CDER) notified ACOG that they will “exercise enforcement discretion” during the ongoing public health emergency with respect to in-person dispensing requirements of mifepristone based on a safety review. Effectively, this will allow providers in states that do not have laws that would otherwise ban this practice to dispense mifepristone using the telehealth protocol for medication abortion.
On May 7th, 2021, in response to the ACLU lawsuit, the FDA announced in a court filing that a review of the REMS is currently underway.
In many parts of the U.S., state laws and policies, requiring some individuals to drive long distances to obtain abortions, sometimes crossing state lines, have increasingly limited abortion access. During the COVID-19 emergency, some states have applied additional restrictions. Some providers, however, have used this crisis as an opportunity to implement new approaches that could potentially expand abortion availability long after the urgency of the pandemic subsides.