A Closer Look at Rape and Incest Exceptions in States with Abortion Bans and Early Gestational Restrictions
Since Roe v. Wade was overturned in June 2022, there has been considerable media attention and legal scrutiny of the health and life exceptions to state abortion bans. This may emerge as an election issue as Former President Trump has stated that he supports rape exceptions to abortion bans, but the 2024 Republican party platform says states should decide their own abortion laws – with no mention of exceptions. The vast majority of Americans – about 8 in 10 – support legal access to abortion for pregnancies resulting from rape or incest. While rape and incest exceptions have been part of the political debate, the feasibility of accessing abortion care under these exceptions has garnered much less attention.
Despite broad support for legal access to abortion in cases of rape or incest, 10 of the 21 states with abortion bans or gestational limits do not have an exception for pregnancies resulting from sexual assault. In the 11 states with rape and incest exceptions, lack of provider availability, law enforcement reporting requirements, and early pregnancy gestational limits can make access to abortion care unattainable for pregnant survivors of sexual assault (Figure 1).
Rape and incest exceptions to state abortion bans or gestational limits are often restricted to abortion early in pregnancy. Among the 21 states with abortion bans or early gestational limits, 11 make exceptions for pregnancies resulting from rape or incest and 10 do not. Of the 14 states with total abortion bans, nine (Alabama, Arkansas, Kentucky, Louisiana, Missouri, Oklahoma, South Dakota, Tennessee, and Texas) lack a rape or incest exception. The remaining 5 (Idaho, Indiana, Mississippi, North Dakota, and West Virginia) have exceptions for cases of rape or incest but limit these exceptions to the earlier stages of pregnancy. Most pregnant people discover they are pregnant at 5.5 weeks LMP, though people living on lower incomes, younger people, Black and Hispanic people, and those experiencing unintended pregnancies often discover their pregnancies later. Of the 7 states with overall gestational limits between 6 and 15 weeks LMP, six (Florida, Georgia, Iowa, Nebraska, North Carolina, and South Carolina) have rape or incest exceptions. Arizona does not have a rape or incest exception in its law.
Most rape or incest exceptions require involvement of law enforcement, which can restrict abortion access for those who have become pregnant as a result sexual assault. In 5 of the states with rape or incest exceptions – Florida, Georgia, Idaho, Mississippi, and West Virginia – pregnant people must report the sexual assault to law enforcement before they can receive abortion care. Many of these states additionally require that the pregnant person provide the physician a copy of the report ahead of receiving care. In Iowa, sexual assault survivors must report the incident “to law enforcement or a public or private health agency which may include a family physician”– within 45 days of the incident (140 days for cases of incest). In South Carolina, survivors are not required to report their assault to law enforcement before receiving abortion care, but physicians who perform abortions under the rape/incest exception must report the allegation of sexual assault to the sheriff in the county in which the abortion was provided.
It is estimated that only 21% of sexual assaults are reported. Survivors are often afraid to report sexual violence to the police due to fear of retaliation and the belief that law enforcement would not do anything to help.
These requirements can also delay care. There are no clear guidelines specifying how quickly law enforcement must issue a copy of the report in these states. Advocates argue it is difficult to get a copy of a police report while the sexual assault is still being investigated. Among the states that require law enforcement reporting, only Idaho specifies that survivors of sexual assault are entitled to receiving a copy of the report within 72 hours of making the request.
Pregnant people may face difficulty finding an abortion provider or securing an appointment. In states with total abortion bans, few abortion providers remain. Providers in states with early gestational limits (and the few remaining providers in states with total bans) may be unwilling to provide abortion care in instances of rape or incest – even when there is an exception – out of fear of prosecution. The sexual assault exceptions often do not specifically outline how physicians can ensure that they will not be prosecuted for providing an abortion that falls under the exceptions. In Idaho, Mississippi, and North Dakota clinics and abortion funds have counseled patients to leave the state to obtain abortion care because they have found that it is easier for people to obtain abortion care out of state than to attempt to receive care in-state under the exception.
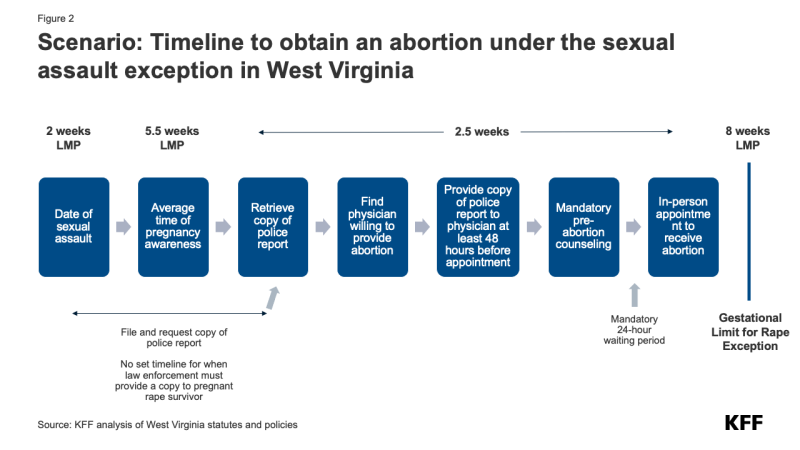
It is instructive to consider a hypothetical scenario in West Virginia, an abortion ban state with a rape/incest exception. The state’s total abortion ban has an exception for pregnancies resulting from rape or incest, but only up to 8 weeks LMP (Last Menstrual Period) for adults, typically about 4 weeks after a missed period. Because most people discover they are pregnant at 5.5 weeks LMP, a person who is pregnant as a result of rape or incest in West Virginia typically would have only 2.5 weeks to:
- Find and secure and appointment with a physician who is willing and able to provide the abortion
- File a report of the assault to law enforcement and retrieve a copy of the report to provide to the physician performing the abortion at least 48 hours before receiving the abortion
- Secure sufficient funding to pay for the abortion
- Manage other logistical challenges, such as arranging childcare and time off work.
West Virginia’s ban is layered on top of the abortion restrictions implemented before the Dobbs decision: a 24-hour waiting period, mandatory counseling, an ultrasound offer, and for minors, parental or legal guardian notification or a judge’s approval. The flow chart below shows how tight this timing is. (Figure 2)
Medicaid coverage for abortion care may be inaccessible, even for enrollees who qualify for a rape or incest exception. Although the federal Hyde Amendment requires state Medicaid programs to cover abortions in cases where the pregnancy resulted from rape or incest, many state Medicaid programs impose pre-authorization requirements and medically unnecessary restrictions. As a result, few, if any, pregnant people obtain Medicaid coverage for their abortion care. For low-income survivors of sexual assault, cost can be an insurmountable barrier to accessing abortion care. In 2021, the median cost of abortion services exceeded $500. However, 43% of women ages 18-49 cannot cover an emergency expense of at least $500 using their current savings, with even larger shares of Black and Hispanic women (57% and 58%, respectively) being unable to cover such an emergency medical expense.
Few people have accessed abortion care under the rape or incest exceptions to state abortion bans. While data from states with abortion bans and exceptions for rape or incest is scant, estimates show that very few abortions are provided in states with total abortion bans, even in those with sexual assault exceptions. For example, Mississippi and Idaho each had 5 documented abortions in 2023, though neither specify the exception under which the abortions were provided. In West Virginia, 23 abortions were provided from January 2023 through June 2024, but none are reported to have been provided under the state’s rape/incest exception. Since Indiana’s total ban went into effect in late August 2023, providers have reported that 5 abortions were provided due to rape or incest. The true number of pregnancies that result from rape is unknown. Given the extremely low number of abortions states have reported as qualifying for rape exceptions to abortion bans and what is known about the high rates of sexual violence that women experience, it would appear that these exceptions have not provided the level of access to abortion for pregnant rape survivors that the laws presumably are designed to protect.