
The independent source for health policy research, polling, and news.
3 Charts: Medicare Drug Price Negotiations
Under the Inflation Reduction Act, the federal government for the first time will negotiate directly with drug companies to determine the prices that Medicare will pay for certain high expenditure drugs covered under Medicare Part D (starting in 2026) and Part B (starting in 2028). Part D covers retail prescription drugs and Part B covers physician-administered medications.
This new requirement is the culmination of years of debate among lawmakers in Congress, and the Centers for Medicare and Medicaid Services is implementing the program according to the timeline established in the law. Meanwhile, the drug industry and others have filed several lawsuits seeking to block the effort. This week CMS faces a deadline to submit proposed prices to manufacturers for certain drugs.
These three charts explain key elements of the drug negotiations process and what it means for Medicare beneficiaries, the timetable according to which the process will unfold, and the public’s views about requiring the government to negotiate drug prices.
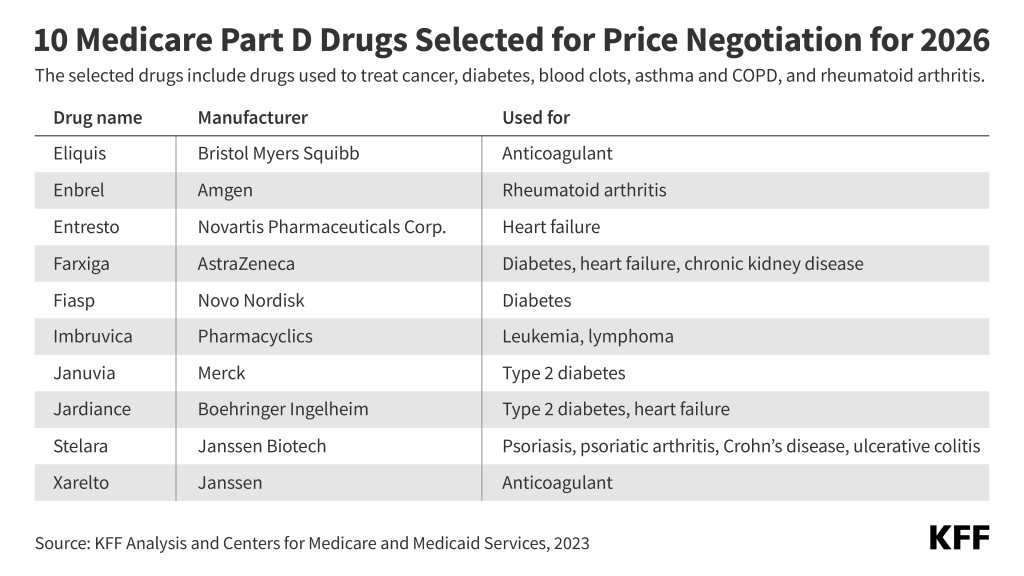
1) The first ten Part D drugs selected for Medicare price negotiation on August 29, 2023 include treatments for diabetes, blood clots, heart failure, psoriasis, rheumatoid arthritis, Crohn’s disease, and blood cancers. Collectively, Medicare spent more than $50 billion on those drugs in a recent 12-month period. The number of drugs subject to price negotiation will increase in future years. The Congressional Budget Office has estimated that negotiated prices will reduce Medicare spending on drugs subject to negotiation, translating into nearly $100 billion in federal savings between 2026 and 2031. Price negotiations also could lower costs for some patients and boost utilization by helping to make these drugs more affordable for Part D enrollees.
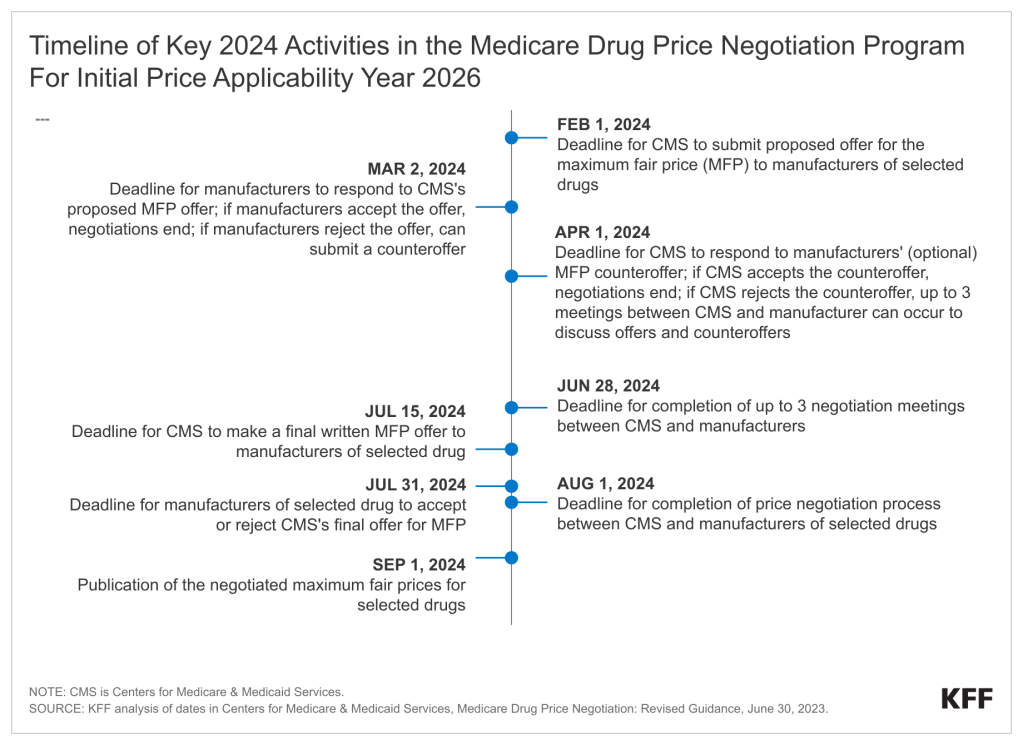
2) Although the Medicare negotiated drug prices for these 10 drugs won’t take effect until January 1, 2026, the price negotiation process between CMS and manufacturers has already started to play out and will continue to do so over the next several months of 2024. On February 1, CMS is required to submit to manufacturers its proposed offer for the “maximum fair prices” – or the negotiated price that CMS is willing to pay – for the selected 10 drugs. The process allows manufacturers to submit a counteroffer and offers the opportunity for additional back-and-forth between the federal government and drug companies over the next several months. The negotiation process ends on August 1 and the final negotiated maximum fair prices for the drugs will be made public on September 1.
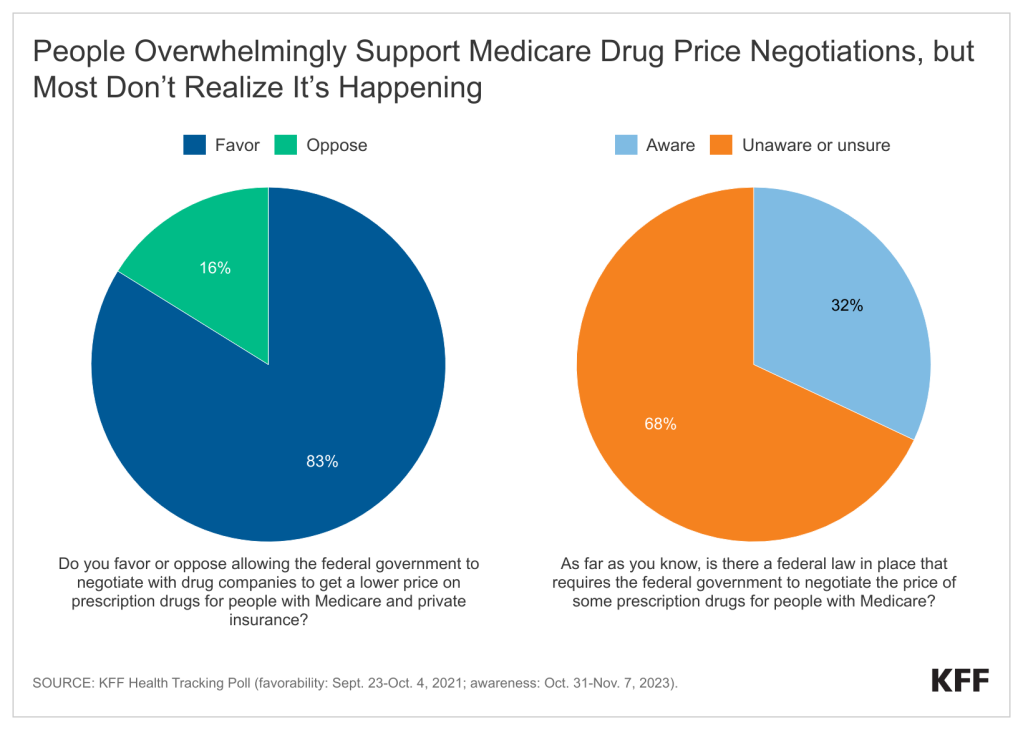
3) The public overwhelmingly supports the idea of allowing Medicare to negotiate drug prices, including majorities of Democrats and Republicans, according to KFF polling. Substantial support among the public persists after hearing arguments for and against the idea. KFF polling also shows that a large majority of people, including most older adults on Medicare, are unaware or unsure that there is a law on the books that requires the government to conduct these negotiations. Moreover, less than half of older adults know about other provisions in the Inflation Reduction Act that could save Medicare beneficiaries money, such as an insulin copay cap for Medicare beneficiaries and new limits on out-of-pocket prescription drug costs for people with Medicare.
For more about the Medicare drug price negotiations process and all of the prescription drug-related provisions in the Inflation Reduction Act, visit kff.org, where you will find the following resources:
- FAQs about the Inflation Reduction Act’s Medicare Drug Price Negotiation Program
- How Medicare’s New Drug Price Negotiation Program Could Expand Access to Selected Drugs
- The New Help for Medicare Beneficiaries with High Drug Costs That Few Seem to Know About
- Explaining the Prescription Drug Provisions in the Inflation Reduction Act