Key Findings
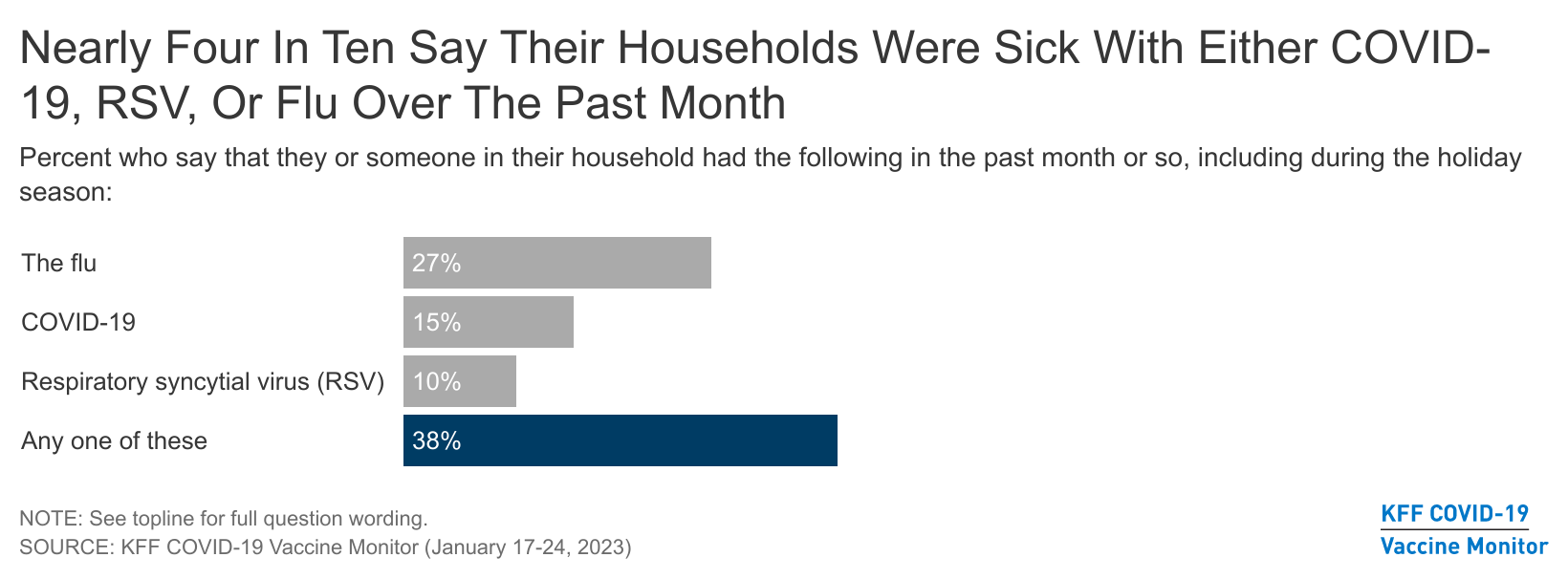
- Nearly four in ten (38%) U.S. adults report that their households experienced either COVID-19, the flu, or RSV over the past month or so and the holiday season, including nearly three in ten (27%) who had someone who was sick with the flu, and one in ten or more who had COVID-19 (15%) or RSV (10%). Three in four (75%) adults in sick households tried to get common over-the-counter medicines like Tylenol or cough syrup when sick, but one in five of those who tried (20%) reported experiencing difficulties getting medicines. Few of those in households sick with COVID-19 or the flu tried to get Paxlovid (14%) or Tamiflu (16%), respectively.
- With the viruses spreading this winter, nearly half of adults (46%) say the news of COVID-19, flu, and RSV have made them more likely to take at least one protective measure. This includes three in ten (31%) who say they are more likely to wear a mask in public, avoid large gatherings (26%), and about one in five who say they are less likely to travel (20%) or eat indoors at restaurants (18%) because of the news of the viruses spreading. Six in ten (63%) immunocompromised people say the news of the viruses spreading has made them more likely to take at least one of these precautions, including four in ten (44%) who say they are more likely to wear a mask in public or avoid large gatherings (40%). While some people report being more likely to change their behavior due to concerns about the viruses, large majorities of those who have not been vaccinated or boosted for COVID-19, or received a flu shot this winter say the viruses spreading have not made much of a difference in their willingness to get these vaccines.
- Despite the continued urging of public health officials for the public to get the updated bivalent booster, available since September 2022, uptake remains modest with about three in ten (28%) saying they have already gotten it, up from 22% in December. The overall rise in the share who say they have gotten the updated booster is driven largely by Republicans, of whom one in five (20%) now say they have gotten it, up from 12% in December. Still, about half of Republicans say they will get the booster only if required (20%) or are not eligible for the booster (31%) due to being unvaccinated or only partially vaccinated. Nearly half (47%) of seniors 65 and older now report having received the updated booster. And about half (52%) of immunocompromised adults say they have received the updated booster (36%) or plan to as soon as possible (16%).
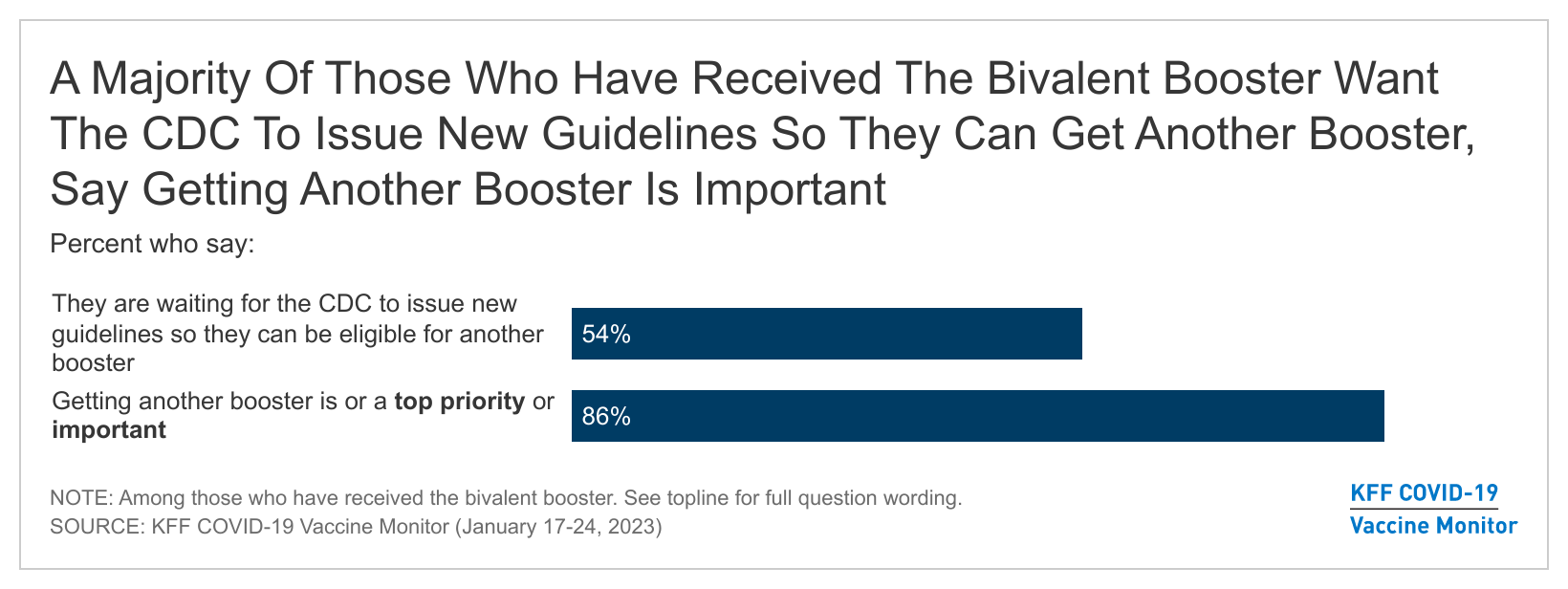
- Many vaccinated adults who are eligible for the updated bivalent booster but have not yet gotten it say they feel they have enough protection from their initial COVID-19 vaccine or a prior infection of the virus (51%), or that they don’t think they need the new booster (44%). But there is still interest in getting an updated dose among some, as about three in ten (29%) say they are too busy or have not had time to get it yet. On the other hand, some are anxiously awaiting the rollout of another booster dose. More than half (54%) of adults who have received the bivalent booster (or 15% overall) say they are waiting on the CDC to issue new guidelines so they can be eligible for another booster. And when asked about getting another booster shot in the future, the vast majority of adults (86%) who have already gotten the bivalent booster say it’s important, including more than one third (37%) who say it is a top priority.
Nearly Four In Ten Adults Say Their Households Were Sick With COVID-19, RSV, Or The Flu During The Holidays And January
While reports have shown a recent decline in hospitalizations for COVID-19, flu, and RSV, the latest COVID Vaccine Monitor survey finds the so-called “tripledemic” was indeed a feature of most Americans lives over the past month or so, including the holiday season. Nearly four in ten (38%) U.S. adults report that their households had someone who was sick with at least one of these three viruses over the past several weeks. About three in ten (27%) adults say someone in their household was sick with the flu, and smaller, though substantial shares experienced COVID-19 (15%), or respiratory syncytial virus (RSV) (10%).

Over the past several weeks, news sources have reported that the country is experiencing a shortage in many common over the counter medicines, such as children’s Tylenol. Most (75%) adults living in households sick with any of the three viruses tried to get over-the-counter medicines to treat their illness, and one in five (20%) of those in households who reported being sick with at least one of the three and tried to get over-the-counter medicines had difficulty getting medicines when they were sick. Overall, 6% of U.S. adults reported difficulty getting over the counter medicines.
The share who said they had a hard time getting over-the-counter medicines is similar across income groups, but parents in sick households who tried to buy medicine are about twice as likely as non-parents (29% vs. 14%) to report difficulty getting medicine.
Few adults in households sick with COVID-19 or the flu tried to get anti-viral treatments that have been shown to reduce the severity and duration of illness. About one in seven (14%) adults in households sick with COVID-19 report they or the person in their house who was sick tried to get Paxlovid, the anti-viral pill used to treat COVID-19, while about three in four (77%) say they did not try to get it. A further one in ten (9%) said they have not heard of Paxlovid.
The January Covid Vaccine Monitor survey finds a similar pattern among those in households sick with the flu with attempts to get Tamiflu, an antiviral medicine used to treat the flu. While 16% of adults in households with the flu said they tried to Tamiflu, about seven in ten (71%) said they did not try to get it, while about one in ten (13%) said they had not heard of Tamiflu.
At Least One In Four Adults Are Worried About Getting Seriously Sick From COVID-19, The Flu, And RSV
As the public health emergency order is set to end, at least a quarter of adults say they are worried about COVID-19, RSV, or the flu. Three in ten (31%) adults say they are “very worried” or “somewhat worried” about seriously getting sick with COVID-19, a slightly larger share than those who said the same of the flu (26%) and RSV (25%). The share who say they are worried about COVID-19 is still somewhat similar to the share (34%) who were concerned in January 2022 amid the surge in the Omicron variant of the virus.
Adults who report being immunocompromised1 , that is that a doctor has told them that they are immunocompromised, or have a weakened immune system due to a disease, treatment, or medication they take are more likely to be worried about getting seriously sick from RSV or the flu than non-immunocompromised adults. But adults who are immunocompromised and those who are not immunocompromised are equally worried about COVID-19. And, younger adults under age 30 are less likely than middle age adults 30-49 to say they are “very worried” or “somewhat worried” about getting seriously sick from COVID-19, the flu, and RSV.

Parents are generally more worried about their children getting seriously sick than adults are about themselves, but parents are equally concerned about their children getting sick from each of the three viruses. Nearly half of parents say they are “very” or “somewhat” worried about their children getting sick with one of these viruses, including 46% who say they are worried about RSV, 44% about the flu, and 41% about COVID-19.
The Tripledemic’s Impact on Behaviors
When asked about the impact of the “tripledemic” viruses spreading this winter on their behaviors, about half of adults (46%) say the news of COVID-19, RSV, and the flu spreading this winter made them more likely to take at least one protective measure, including wearing a mask in public, avoiding large gatherings, traveling less, or avoiding dining indoors at restaurants. Of all the measures asked about, wearing a mask in public is the most often reported protective measure taken, across ages, race/ ethnicity, and partisanship. Three in ten adults (31%) say the viruses spreading have made them more likely to wear a mask, followed closely by avoiding large gatherings (26%). About one in five adults say the “tripledemic” has made them less likely to travel (20%), or to eat indoors at restaurants (18%).
The CDC advises immunocompromised individuals to take extra precautions to protect themselves from COVID-19 and other pathogens. The January COVID-19 Vaccine Monitor finds that nearly two-thirds (63%) of adults who say they are immunocompromised have modified at least one of their behaviors to be more cautious. And on each of the measures asked about, immunocompromised individuals are more likely to have taken a precautionary measure than those who are not immunocompromised, with the exception of indoor dining. Still, a substantial share (45%) of adults who are not immunocompromised have modified at least one of their behaviors as well.
Adults ages 65 and older are significantly more likely than their younger peers to say they have taken at least one of the precautions this winter. Nearly six in ten (58%) older adults say they have modified at least one of these behaviors, including 43% who say they are more likely to wear a mask in public and one in three (34%) who said they more likely to avoid large gatherings.
Black adults and Hispanic adults are more likely than White adults to say they have taken at least one of these precautionary measures. Two-thirds of Hispanic adults (67%) and Black adults (67%) say they have modified their behaviors to be more cautious, while about four in ten (38%) of White adults say the same.
Democrats are more than twice as likely as Republicans to say the viruses spreading this winter have made them more likely to take at least one of these precautionary measures (64% vs. 28%). Continuing the trend of partisanship and mask-wearing, half of Democrats (46%) say following the news of the “tripledemic” that they are more likely to wear a mask in public, compared to just one in eight (16%) Republicans who say the same. A similar partisan divide is seen on each of the other measures tested, with Democrats being twice as likely or more to say they are changing their behavior compared to their Republican counterparts.
Uptake Of The Updated Bivalent COVID-19 Booster
Although about half of adults (46%) say they are taking at least some protective measures given the news of COVID-19, RSV, and the flu spreading in the U.S. this winter, a smaller share of adults are doing the thing public officials are emphasizing the most: getting the updated bivalent booster. About four in ten adults say they have either received the updated bivalent COVID-19 booster dose (28%)2 or that they plan to get it “as soon as possible” (14%). In a slight uptick from last month’s Covid Vaccine Monitor, nearly three in ten (28%) adults now say they have already gotten the updated booster, up from 22% in December. Still, about one in ten adults say they want to “wait and see” before getting the updated shot (12%), and the same share (12%) says they will get it “only if required.” A further 11% say they will “definitely not” get the updated booster and about one in five (22%) adults are not eligible for the updated dose since they are unvaccinated or only partially vaccinated.

The Biden administration recently announced plans to target senior care facilities to further encourage uptake of the new booster among older adults. Nearly half (47%) of adults ages 65 and older say they have already gotten it, and a further one in ten (12%) say they plan to get it “as soon as possible.” Though relatively few (3%) say they will only get the updated vaccine if required, a further one in five adults 65 and older say either say they will “definitely not” get the updated booster (12%) or remain ineligible to receive it (11%), leaving a sizeable share of this vulnerable group without the protection of the vaccine.
Adults who say they are immunocompromised are no more likely to report that they have already received the updated bivalent booster than those who do not report being immunocompromised. About half of adults (52%) who say they are immunocompromised say they have either received the updated bivalent COVID-19 booster dose (36%) or that they plan to get it as soon as possible (16%).
The share of Democrats who have received the updated shot continues to outpace both Republicans and independents. However, the share of Democrats who say they have already gotten the booster (39%) has not changed since December (38%), while Republican uptake has increased. One in five Republicans (20%) now say they have gotten the updated booster compared to about one in ten last month (12%). Despite this uptick, overall Republican opposition to the booster remains staunch, as about half of the partisan group either say they will “definitely not” get the updated booster (20%) or are ineligible (31%) for the booster because they are either unvaccinated or are only partially vaccinated.

Those who say they are worried about getting seriously sick from COVID-19 are more likely to have already received the updated bivalent booster (40%) than those who say they are not worried (23%). However, for the most part, large majorities of those who have not been vaccinated or boosted for COVID-19, or received a flu shot this winter say the news of COVID-19, RSV, and the flu spreading this winter have not made much of a difference in their willingness to get these shots. Nearly nine in ten (87%) of those who are unvaccinated say the news has not made much of a difference in their likelihood of getting a shot. A similar share (82%) who have not gotten a flu shot for the current season also say the news has not made much of a difference in their likelihood to get a flu vaccine. However, about one in five adults (22%) who are vaccinated but haven’t gotten a COVID-19 booster dose say the news of the viruses spreading has made them more likely to get a COVID-19 booster.
Reasons For Not Getting The Updated Bivalent Booster
Adults who are vaccinated but have not yet received the updated bivalent booster cite a range of reasons for why they have not yet done so. About half (51%) say they feel they have enough protection from their initial COVID-19 vaccine or a prior infection of the virus, and about four in ten (44%) say they don’t think they need the new booster. Reflecting that there is still some interest in getting an updated dose, about three in ten (29%) say they are "too busy” or “have not had time to get it.” Other less frequently cited reasons include the nearly one in five (19%) of adults who say they had bad side effects from a previous COVID-19 dose, or they cannot afford to take time off work to get the booster or deal with the side effects of the vaccine (15%). Similar shares say they have not gotten an updated bivalent booster because they think “COVID is over” (15%), while about one in ten (12%) say they are not sure how or where to get the booster.
Reasons for not getting an updated booster shot vary little across age groups, though there are some notable exceptions. Compared to older adults, younger adults (ages 18-29) are significantly more likely to say they are too busy or have not had time to get it, or they had bad side effects from a previous dose. Despite the push from public health officials to boost adults 65 and older, half (50%) of vaccinated adults in this age group say they feel protected enough from a previous dose or prior infection, and nearly four in ten (38%) say they don’t think they need the new booster.

When asked in their own words for any other reason why they have not gotten a booster dose yet, few responses covered topics not already asked about explicitly. The most frequent cited other responses among those who are eligible but have not been boosted are concern about possible side effects of vaccination (7%). Less frequently mentioned responses include 4% say they distrust the vaccine, drug companies or the government, don’t think the vaccine is effective (3%), they need to wait to get a booster due to a recent COVID-19 infection or booster (3%), or didn’t know about the booster (1%). Another 5% gave a host of other reasons ranging from they are unclear if their insurance will cover the shot or not, to wanting to strengthen their immune system by other means, to that they think they are at low risk of complications from getting COVID.
In their own words: “Is there any other reason why have you not gotten another booster shot?”
“Covid is a virus, like the flu, that will be a part of our lives. I do not get flu vaccines and have chosen healthy ways to recover naturally and build natural immunity.” -50 year-old, female, Hispanic, Republican, Florida
“I didn’t know anything about it until now” –18 year-old, male, Hispanic, independent, California
“Unclear on insurance coverage” – 29 year-old, female, White, Democrat, Missouri
“I’ve had it twice now with no symptoms. I believe I don’t need it and am at low risk of complication from COVID.” – 40 year-old, female, White, Republican, Arizona
“I’ll get it if it’s extremely necessary” – 49 year-old, female, Hispanic, independent, Florida
“I am young and healthy and when I previously had COVID it was manageable. Additionally, I have the option to work remote so wouldn’t affect my job If I were to get it.” – 28 year-old, female, White, Democrat, Minnesota
“I keep forgetting to get it, I also don’t leave the house much so there’s a very small chance I catch it.” –20 year-old, male, Hispanic, other party, Ohio
The top reasons given by those eligible for the new booster who have not yet gotten it are similar across partisans, but the share who say each reason varies. Majorities of Republicans or Republican-leaning independents say they have not gotten the booster because they feel they have enough protection from a previous dose or infection (62%), or they don’t think they need it (56%). These are also the top reasons given by Democrats and Democratic-leaning independents, but four in ten of this group (43%) say they feel they have enough protection and about one in three (34%) say they don’t think they need the new booster. Other reasons are more frequently mentioned by Democrats and Democratic-leaning independents than Republicans, including being too busy or not having time (37% vs. 17%), that they can’t afford to take time off work to get the booster or deal with side effects (19% vs. 10%), or they are not sure how or where to get the booster (15% vs. 6%). Notably, one-fourth (27%) of Republicans say they have not gotten a booster because they think “COVID is over.”

More Than Eight In Ten Boosted Adults Say It’s Important For Them To Get Another Booster, And More Than Half Say They Are Waiting On The CDC To Issue New Guidelines
While studies continue to investigate the longevity of the new updated bivalent booster, some consensus has emerged that immunity and protection against infection is likely short-term. The January COVID-19 Vaccine Monitor survey finds many who have already received the new booster are now eagerly awaiting guidance from the CDC about when they can be eligible for another shot. More than half (54%) of adults who have received the bivalent booster say they are waiting on the CDC to issue new guidelines so they can be eligible for another booster. Overall, 15% of U.S. adults say they are waiting on the CDC to issue new guidelines.

When asked about getting another booster shot in the future, the vast majority of adults (86%) who have already gotten the bivalent booster say it’s important, including more than one-third (37%) who say it is a “top priority” and half (49%) who say it is “important but not a top priority.” A further one in ten (10%) say it is “not too important,” while 3% say it is something they don’t plan to do.
When asked why getting another booster shot is important to them, nearly all those asked provide responses related to the fact that they want to protect themselves from the virus. About one in four (24%) offer reasons like they want to get another shot to prevent getting COVID, to stay healthy, or avoid getting sick. Another 7% offer responses about wanting to protect themselves as much as possible, while a further 7% say they want another shot because they are immunocompromised, elderly, or in a high-risk group.
Other responses given about why getting another booster is a top priority or important but not a top priority touch on why boosted adults are not eager to get another one. About one in seven (14%) offer responses like they have already gotten multiple boosters and thus felt protected with the ones they have received thus far. Smaller shares gave other responses like that they don’t think COVID is that bad (6%), that it is a “mild disease.” Relatively few (13%) adults who have received the updated, bivalent booster say getting another shot is not too important or something they don’t plan to do, and explanations for why ranged from that they only get vaccinated as a condition of employment, to that they are unsure how effective the new booster is.