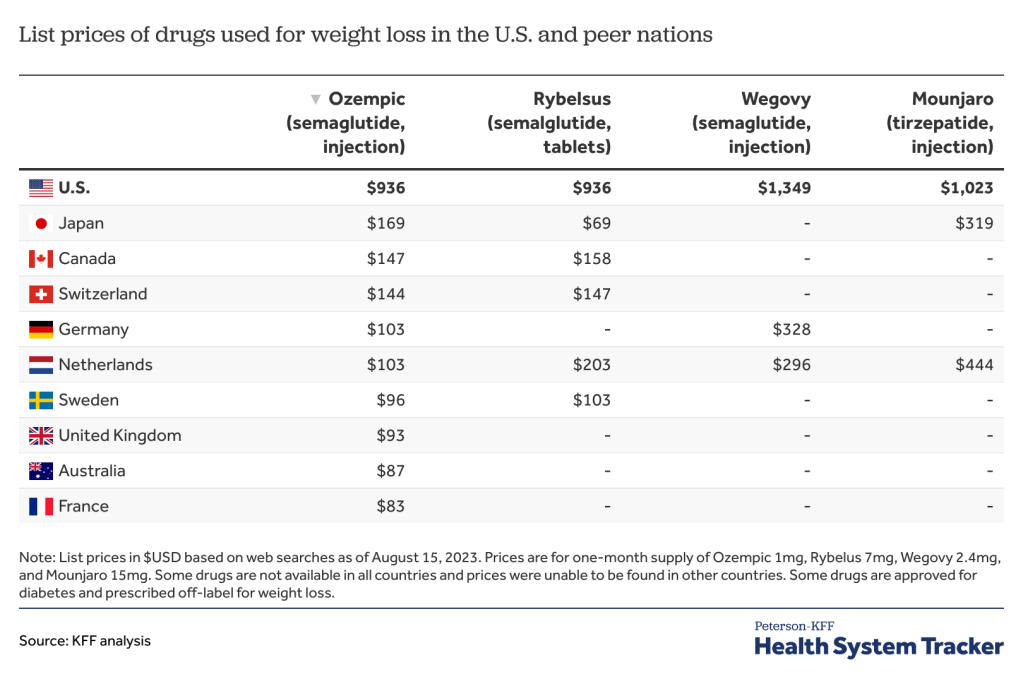
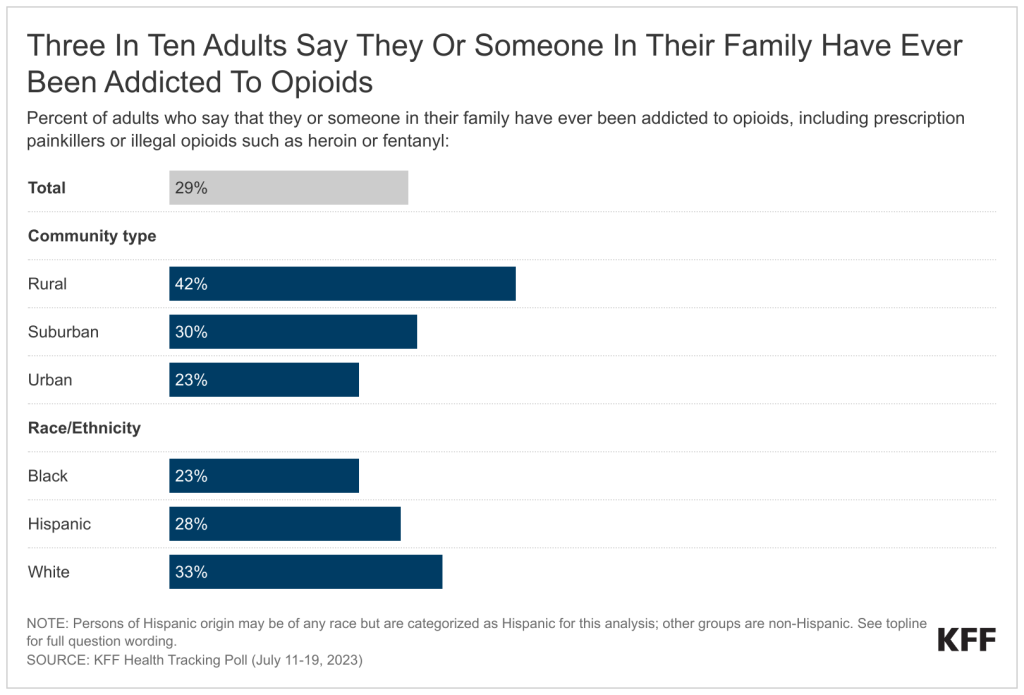
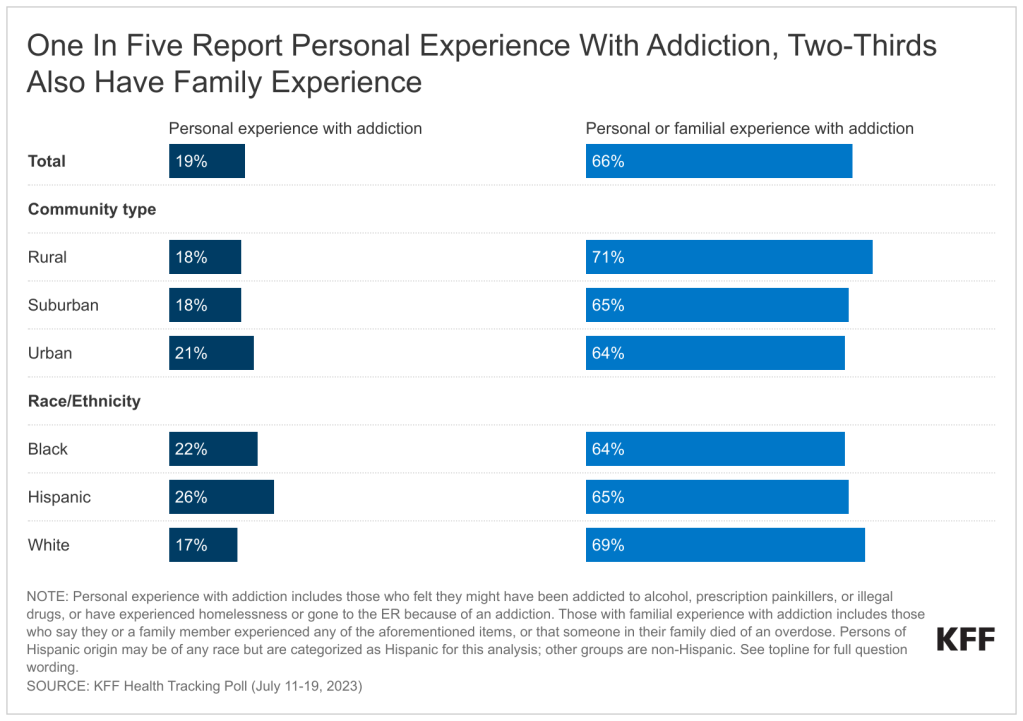
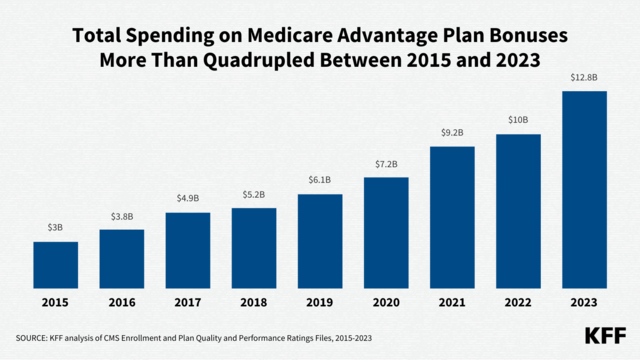
Policymakers and the media have been increasingly attentive to mergers and acquisitions and other potentially anticompetitive practices of hospitals, physicians, and other health care providers. Consolidation has the potential to increase efficiency and help some struggling providers to keep their doors open in relatively underserved areas, but it can also reduce market competition and ease pressure on providers to lower prices or invest in quality improvements. A substantial body of evidence shows that consolidation has led to higher prices without clear evidence of improvements in quality, which has implications for consumers and employers. As a result, some have proposed strengthening antitrust regulation—which aims to protect competitive markets—as a tool for tackling rising health care costs, increasing the affordability of care, and reducing the large number of adults with medical debt.
Federal and state antitrust agencies play a role in challenging anticompetitive practices of health care providers and other businesses. At the federal level, the Federal Trade Commission (FTC) and the Department of Justice (DOJ) share responsibility for enforcing federal antitrust laws, including the Sherman Act, the Clayton Act, and the FTC Act. State attorneys general (AG) offices also have the authority to bring action under federal antitrust law, as well as under state statutes, which sometimes expand upon federal law.
This issue brief explains the role of federal and state antitrust agencies in challenging anticompetitive practices among health care providers, including the legal authority of federal and state agencies, the role that they play in enforcing antitrust laws, and proposed options for strengthening their authority. The brief focuses on health care providers, though many of the principles discussed in this issue brief apply to the practices of other health care entities as well, such as health insurers and pharmacy benefit managers (PBMs) (which are currently being reviewed by the FTC). While the focus of this brief is on the role of government agencies, antitrust law also authorizes private parties, such as employer health plans, to challenge anticompetitive practices in the courts.
What types of anticompetitive practices do governments challenge and why?
Governments challenge anticompetitive practices to promote competitive markets, often for the benefit of consumers (e.g., patients and health plan enrollees).1 Governments seek to address a variety of anticompetitive practices that may lead to higher prices without commensurate improvements in quality of care. These include anticompetitive mergers and acquisitions (referred to as “mergers” in this brief), and other activities that hinder competition (referred to as “nonmerger anticompetitive practices” in this brief).
Provider consolidation can be beneficial to consumers in some instances and detrimental in others. On the one hand, consolidation has the potential to increase efficiency, such as by allowing providers to purchase supplies in bulk at a discount or by facilitating the coordination of care across different providers. On the other hand, consolidation has the potential to lead to worse outcomes for consumers by increasing providers’ market power and decreasing competition, which enhances the ability of providers to negotiate for higher prices (increasing costs for consumers and employers) and reduces the pressure on providers to invest in quality improvements. A substantial body of evidence shows that consolidation has led to higher prices without clear indications of quality improvements, though the strength of this evidence varies based on the type of consolidation and provider.
There are three main types of mergers:
- Horizontal mergers occur when there is consolidation between providers that offer the same or similar services, such as when a health system acquires a hospital or when two physician practices that provide overlapping services merge. Horizontal mergers can raise concerns about competition because they, by definition, reduce competitiveness when occurring between providers in the same market, and because consolidated entities can take actions to increase and protect their market power.
- Vertical mergers occur when there is consolidation between providers that offer different services along the same supply chain, such as when a hospital acquires a physician practice. Vertical mergers can raise anticompetitive concerns, for example, if physicians refer patients to hospitals within their health system rather than to competing hospitals. Some mergers may entail both vertical and horizontal consolidation (e.g., if a health system acquires a physician group that provides services offered by the system’s existing physician group).
- Cross-market mergers occur when there is consolidation between providers that operate in different geographic markets.2 Cross-market mergers may raise concerns about competition, for example, if a health system with providers in different areas of a state is able to use its dominant position in one market to negotiate higher prices in another when contracting with a given health plan (e.g., a state employee plan with enrollees that reside in several markets).
Governments also challenge other types of anticompetitive practices, such as the use of anticompetitive clauses in contracts between providers and insurers or providers and workers. Anticompetitive contract clauses give dominant parties an unfair advantage over potential competitors and can lead to higher prices. For example, some health systems have highly regarded hospitals (also known as “must have” hospitals) that insurers need to include in their provider networks in order to attract enrollees, which gives these systems substantial bargaining leverage over insurers. These health care systems can in turn use this bargaining leverage to pressure insurers to contract with all providers in the system (through “all-or-nothing clauses”), shielding expensive or low-quality members from competition with more desirable providers. The textbox below provides definitions of various anticompetitive contract clauses.
Common Types of Anticompetitive Contract Clauses3
- All-or-nothing clauses require an insurer that wants to contract with a particular provider in a system (such as a must-have hospital) to contract with all providers in that system.
- Anti-tiering/anti-steering clauses prevent an insurer from putting a given provider in a non-preferred provider network tier or from using other incentives or tools to steer patients to competing providers. This can incentivize patients to use that provider, even if a higher-value provider is also in-network.
- Exclusive contracting clauses prohibit an insurer from including competing providers in their provider network, so that a given provider is the only in-network option in a given area.
- Non-compete clauses prevent a worker employed with a given provider from taking a job with a competing provider or starting a new practice within a certain distance for some duration of time.
- Most favored nation clauses require a provider to offer an insurer the lowest rates of all the insurers with which it has contracted. While the examples above create favorable terms for providers in their contracts with insurers, most favored nation clauses create favorable terms for insurers in their contracts with providers.4
What federal antitrust laws govern anticompetitive practices?
There are three primary federal antitrust laws—the Sherman Act, the Clayton Act, and the FTC Act—that prohibit anticompetitive mergers and other anticompetitive practices.
- The Sherman Act (1890) broadly prohibits anticompetitive practices. It has been used to challenge various anticompetitive practices, such as mergers, wage suppression, agreements among competing businesses to fix prices, and anticompetitive contracting clauses.
- The Clayton Act (1914) builds on the Sherman Act by explicitly prohibiting anticompetitive mergers as well as other types of anticompetitive practices that are not clearly addressed by the Sherman Act (such as by barring the same individual from serving on the board of directors for two competing health systems, with some exceptions). Additionally, as amended under the Hart-Scott-Rodino Act in 1976, the law requires that merging entities report their plans in advance to federal regulators in certain cases where the transaction exceeds a specified value ($111.4 million in 2023), which gives regulators time to investigate and intervene if needed.
- The Federal Trade Commission (FTC) Act (1914) created the FTC and prohibits “unfair methods of competition” and “unfair or deceptive acts or practices.” The FTC Act encompasses the same types of violations that are covered by the Sherman Act and the Clayton Act, in addition to other anticompetitive practices, and grants the FTC regulatory authority. Unlike the Sherman Act and the Clayton Act, the Act generally cannot be applied to nonprofit entities.5
Some forms of business practices, such as almost all instances where competitors coordinate to raise prices, are inherently illegal under federal law. The legality of other types of business practices depends on the context. For instance, when government agencies challenge a merger, courts assess whether the merger would likely harm competition in a given market.
What is the FTC’s role in enforcing federal antitrust law?
Two federal agencies—the FTC and DOJ—have overlapping, as well as distinct, authority to challenge anticompetitive practices under federal law. The FTC is the only entity that can enforce the FTC Act. Although this Act generally cannot be applied to nonprofit entities, the FTC has the authority to enforce the Clayton Act against nonprofit entities (in addition to for-profit entities), such as by challenging anticompetitive mergers among nonprofits. The DOJ has the authority to enforce the Sherman and Clayton Acts. States can also bring lawsuits under federal antitrust law, as can some private parties, such as competing providers.
The FTC focuses on “protecting the public from deceptive or unfair business practices and from unfair methods of competition.” This includes challenging activities such as misleading advertisements, violations of consumers’ data privacy, and efforts to accumulate market power through mergers and other anticompetitive practices. For instance, the FTC sued Facebook in 2020, alleging, in part, that the company had sought to maintain its monopoly power by buying up competitors, such as Instagram and WhatsApp.
The FTC plays a larger role than the DOJ in enforcing federal antitrust law in health care provider markets, though there are gaps in its authority. The FTC and DOJ have each developed expertise in certain areas and have tended to divide merger oversight accordingly, with the FTC typically overseeing provider markets and the DOJ typically overseeing insurance markets (see more below). However, although the FTC has broad authority to challenge anticompetitive mergers, its authority to challenge other anticompetitive practices often excludes nonprofits. Nonprofit ownership is common in provider markets. For example, nonprofits account for about three-fifths (58%) of community hospitals in 2023. The DOJ may fill in for the FTC when the FTC does not have the authority to challenge nonprofit providers that are engaging in certain anticompetitive practices (see example of Atrium Health below).
The FTC has successfully challenged several hospital mergers over the past two decades. Beginning in the 1990s and for several years afterwards, the FTC had difficulty challenging hospital mergers in the courts, allowing rapid consolidation in the hospital sector to continue unabated.6 Since the late 2000s, the FTC has since been more successful in challenging hospital mergers, reflecting advances in both economics and the FTC’s new legal strategies.7 However, the FTC challenges only a fraction of hospital mergers, and it is difficult to know the extent to which mergers that go unchallenged have an adverse impact on consumers, in terms of costs and quality. For instance, in 2022, the FTC challenged 3 hospital mergers and, in each case, the hospitals abandoned their plans to merge, while one analysis documented 53 hospital merger announcements in that year. The same analysis identified more hospital merger announcements in the years prior to the start of the COVID-19 pandemic (e.g., 92 in 2019).
Example: FTC & Advocate Health Care Network
In 2015, the FTC brought a legal challenge against a proposed merger of two Chicago-area health systems: Advocate Health Care Network and NorthShore University Health System. The FTC argued that the combined entity would control over half of the market for general acute care inpatient hospital services, compared to the next largest provider, which would have only controlled 15% of the market. The court placed a temporary block on the merger, and the systems ultimately abandoned their plans to merge before the case went to trial.
The FTC has played a smaller role in challenging physician mergers. A key challenge is that physician mergers tend to be smaller, and physician groups often grow slowly over time by acquiring small group practices and hiring new physicians. As a result, physician groups often do not need to report mergers to federal regulators as their transactions tend to fall below Hart-Scott-Rodino reporting thresholds (though regulators can still challenge mergers that they learn about in other ways). Additionally, because they tend to be small, any given merger may not have an appreciable effect on market power, even if the cumulative effect of these mergers leads to a large concentration of market power over time.
Vertical mergers in health care provider markets have largely escaped FTC enforcement and the FTC has never challenged a cross-market merger, though it has expressed interest in both practices. For instance, in 2021, the FTC announced that it would begin to study the effect of vertical mergers between health care facilities and physician groups. The FTC previously conducted studies of horizontal mergers in advance of successful litigation. The FTC has also investigated specific instances of cross-market mergers, although it has yet to bring a challenge.
Example: FTC & St. Luke’s Health System
In 2012, St. Luke’s Health System in Idaho attempted to acquire Saltzer Medical Group, a physician practice group. Although this proposed acquisition had elements of a vertical merger, the FTC challenged it as a horizontal merger, i.e., on the basis that St. Luke’s would obtain a dominant market share for adult primary care physician services. Courts ruled in favor of the FTC and ordered that St. Luke’s divest Saltzer Medical Group. This was the first time that the FTC received a court decision for a case challenging a hospital or health system’s acquisition of competing physician practices.
The FTC has not played a large role in overseeing nonmerger anticompetitive practices in nonprofit health care provider markets, such as the use of anticompetitive contract clauses. This may reflect the fact that the FTC’s authority to challenge and regulate nonmerger anticompetitive practices generally excludes nonprofit entities. Nonetheless, the FTC has brought legal challenges in some instances, such as cases where separate physician groups have coordinated with each other to raise prices. Relatedly, the FTC drew on its regulatory authority when it proposed a rule in 2023 that would ban non-compete clauses between employers and workers.
What is the DOJ’s role in enforcing federal antitrust law?
The DOJ enforces a wide range of laws on behalf of the federal government, including—through its Antitrust Division—anticompetitive practices. For instance, in one landmark antitrust case in the 1990s, the DOJ sued Microsoft, alleging that the company had illegally sought to protect its monopoly power. The DOJ argued that the company had done so, in part, by requiring computer manufacturers that wanted to use Microsoft’s popular Windows operating system to also include Internet Explorer as a default.
Although the FTC typically oversees the conduct of health care providers, the DOJ has occasionally done so as well (see example below).
Example: DOJ & Atrium Health
In 2016, the DOJ filed a lawsuit against Carolinas Healthcare System, also known as “Atrium Health.” The DOJ claimed that Atrium had violated federal antitrust law by, among other things, entering into contracts with insurers that contained anti-steering and anti-tiering clauses. Atrium Health system and the DOJ reached a settlement agreement before trial where the system agreed, in part, to stop using these contract clauses.
The DOJ typically takes the lead in promoting competition in health insurance markets. For instance, in 2017, the DOJ, along with some state governments, successfully prevented a proposed merger between Anthem and Cigna—which would have been the largest merger of health insurance companies on record—and a proposed merger between Aetna and Humana.
How do the FTC and DOJ work together on antitrust issues?
The FTC and DOJ have a clearance process to determine which agency will investigate and challenge a given merger. The FTC and DOJ have each developed expertise in different areas and have tended to divide merger oversight accordingly, with the FTC typically overseeing provider markets and the DOJ typically overseeing insurance markets. The division of labor is formalized through a clearance process that determines which agency will investigate a proposed transaction based on its expertise and other factors, such as its capacity and ties to a given case.
The FTC and DOJ collaborate on guidelines that establish how they determine whether to challenge a given merger. This includes the Horizontal Merger Guidelines, which, as the name suggests, outline the criteria that the FTC and DOJ consider when reviewing horizontal mergers. For instance, the guidelines indicate that the agencies evaluate the effects of a merger on market concentration based on a measure known as the “Herfindahl-Hirschman Index” (HHI) (see textbox below). The guidelines also indicate that the agencies consider the possible benefits of a given merger, such as whether a merger might allow research to be conducted more effectively.
Herfindahl-Hirschman Index (HHI)
The HHI is calculated based on provider market shares for a given product—such as inpatient general acute care services or inpatient orthopedic surgical services—and geographic market. The HHI for a market can range from nearly 0 (a perfectly competitive market) to 10,000 (a market with a single provider). The Horizontal Merger Guidelines define the level of market concentration as follows:
Unconcentrated: HHI < 1,500
Moderately concentrated: HHI between 1,500 and 2,500
Highly concentrated: HHI > 2,500
Although markets for inpatient hospital services are now often highly concentrated, there is wide variation across the country. For instance, one study estimated that, in 2021, the New York City metro area had an HHI of 753 for inpatient hospital services, while the Wilmington, North Carolina metro area had an HHI of 7,600.
The FTC and DOJ have also released a set of Vertical Merger Guidelines, though the FTC withdrew from these guidelines in 2021. Some economists are more critical of the Vertical Merger Guidelines than the Horizontal Merger Guidelines, perhaps reflecting the fact that there is less consensus about the effects of vertical consolidation and the proper role of antitrust enforcement.
In July 2023, the FTC and DOJ released a draft version of their updated merger guidelines, which would apply to both horizontal and vertical mergers and which indicate that the agencies will be scrutinizing a broader range of mergers. Among other changes, the draft guidelines expand the definition of highly concentrated markets, rely on a lower threshold for identifying large changes in market concentration, consider the combined effect of a series of acquisitions (e.g., of a health system acquiring several small physician practices over time), and add an explicit discussion of the agencies’ views on how workers may be negatively impacted when their employers merge. These guidelines might also be used to challenge cross-market mergers, although this is not yet clear. The deadline for public comments on the draft guidelines is September 18, 2023.
In addition to working together on general merger guidelines, the DOJ and FTC have in the past collaborated on antitrust policy statements that are specific to health care, though both agencies withdrew from these statements in February 2023 and July 2023, respectively, arguing that the statements are outdated based on changes in health care markets.8
What is the role of states in enforcing antitrust law?
States can bring legal challenges under federal antitrust law. States may do so through their AG offices as either a purchaser of health care (for instance, through state employee health plans) or on behalf of their residents. States sometimes file lawsuits jointly with each other or with the federal government, which can help overcome resource constraints. States and the federal government may play complementary roles, with the federal government providing greater resources and general antitrust expertise and states providing more specialized knowledge of local market conditions.
Most states have passed laws that expand oversight of provider mergers, which may lead to additional legal challenges. Thirty-four states and DC require that at least some hospitals notify state AG offices of their plans to merge, expanding on federal reporting requirements. For instance, Rhode Island requires all hospitals to do so, regardless of the value of the transaction. Additionally, thirteen states and DC require that some or all types of providers receive approval from the government prior to merging, instead of requiring that the government file a lawsuit to challenge a merger. Eleven states require AG offices to consider relatively expansive criteria when reviewing health care mergers. For instance, California law requires the AG office to consider criteria such as the general public’s interest and the effect of a merger on access to care.
Some states have prohibited certain types of anticompetitive contract clauses. These laws either broadly prohibit a given type of clause or ban their use in only specific circumstances. Regarding contracts between insurers and dominant providers, two states (Massachusetts and Nevada) have laws restricting at least some all-or-nothing clauses and anti-tiering or anti-steering provisions, and five states (Massachusetts, Minnesota, Nevada, New Hampshire, and Wisconsin) have laws restricting exclusive contracting. Additionally, 22 states restrict non-compete provisions—which dominant providers sometimes use in contracts with their workers—and 20 states restrict most favored nation clauses, which dominant insurers sometimes use in their contracts with providers.
Example: California & Sutter Health
In 2018, the California AG joined a lawsuit that had been initiated on behalf of some group health plans against Sutter Health, a large nonprofit health system in the state. The parties argued that Sutter had used anticompetitive contract clauses—such as all-or-nothing and anti-tiering provisions—to increase prices. In 2019, Sutter agreed to a settlement agreement that required the system to abandon the relevant contract clauses and to pay $575 million in damages, among other things.
Some states have enacted laws that have the potential to shield health care providers from antitrust scrutiny in certain instances. For example, 19 states have Certificate of Public Advantage (COPA) laws, which immunize a merger from antitrust challenges while directly regulating the merged entity for a period of time, such as by limiting price increases or prohibiting certain contracting practices. The intent of COPA laws is to facilitate mergers that are perceived as being beneficial overall while mitigating anticompetitive concerns through state oversight. However, the FTC and some researchers have been critical of these laws, arguing, for example, that states have not followed through in providing ongoing oversight following a given merger. Other state policies, such as Certificate of Need (CON) statutes—which attempt to reduce costs by restricting, for example, the construction of new facilities when they do not meet a community need—may also play a role in limiting competition or preventing antitrust scrutiny.
What are the potential remedies in antitrust enforcement?
Federal or state agencies challenging a merger can seek structural remedies or conduct remedies (also known as “behavioral remedies”).
- Structural remedies mitigate consolidation by preventing a merger from moving forward, breaking up mergers that have already taken place, or requiring a merged entity to sell off a portion of its business.
- Conduct remedies entail restrictions or requirements imposed on providers after a merger, such as by limiting the prices providers can charge, prohibiting providers from engaging in certain contracting practices, or requiring providers to spend a minimum amount on community benefits.
Conduct remedies may be less effective than structural remedies in certain circumstances, as they tend to be time-limited and government agencies may not have the resources to monitor and enforce them. However, where markets are already concentrated and regulators are reluctant to break up merged entities, conduct remedies may be the only option.
When the government challenges proposed mergers before they occur, the recourse is typically to prevent the merger from moving forward. Antitrust enforcers have only infrequently attempted to unwind mergers that have already taken place, which the FTC describes as a “difficult and potentially ineffective” process.
There are other ways in which the government can be successful in challenging anticompetitive mergers. For example, the government and merging providers may avoid trial through a settlement agreement or consent decree. In this scenario, the government drops its legal challenge in exchange for structural or conduct remedies. Additionally, providers may abandon a merger after a lawsuit is announced or a court makes a preliminary ruling against the merger or may decide not to attempt to merge in the first place in anticipation that doing so would be successfully challenged in court.
Example: FTC & Phoebe Putney Health System
In 2011, Phoebe Putney Health System acquired a hospital from HCA in Albany, Georgia. The FTC challenged the merger and eventually reached a settlement agreement with the providers. The settlement agreement allowed the merger to persist but imposed conduct remedies, including that Phoebe Putney notify the FTC before acquiring other health care providers in the area.
The outcomes of successful legal challenges to nonmerger anticompetitive practices are similar, though the remedy would involve abandoning the relevant business practice (e.g., no longer using anticompetitive contract clauses).
What are some practical challenges facing antitrust enforcement?
There are at least a few challenges that may limit the ability of the federal government and states to foster competitive provider markets through antitrust enforcement:
- It is difficult to break up mergers after they have already occurred, and many provider markets are already highly concentrated. For example, one study estimated that the vast majority (90%) of metropolitan statistical areas (MSAs) had highly concentrated hospital markets in 2016 (i.e., with an HHI above 2,500), most (65%) had highly concentrated specialist physician markets, and nearly two in five (39%) had highly concentrated markets for primary care physicians. Breaking up a merger after providers have already consolidated can be difficult. At the same time, regulating the behavior of merged providers—such as through restrictions on the prices they charge—may be difficult to do on an ongoing basis.
- Some regions cannot support competitive provider markets. For instance, rural communities may not have enough residents to support several providers that offer the same service.
- Antitrust litigation can be complex and expensive. Without adequate funding, it may be impractical to challenge a large number of provider business practices that raise anticompetitive concerns.
- Antitrust agencies may have difficulty staying ahead of market trends. For example, it could take time for the government to develop strong guidelines for challenging vertical or cross-market mergers and to accumulate enough evidence to convince courts that these practices harm competition. In the meantime, these mergers will likely continue.
- The benefits of competitive provider markets for individuals with health insurance will depend in part on the competitiveness of health insurance markets. The study referenced above also estimated that most MSAs (57%) had highly concentrated insurance markets in 2016. When insurance markets are not competitive, cost savings from competitive provider markets might not be fully passed along to consumers.
What policies have been proposed to strengthen antitrust law and enforcement?
Several federal and state policy proposals have been floated to help antitrust regulators more easily identify and challenge anticompetitive mergers and regulate markets that are already concentrated. One set of policies would make it easier for governments to enforce antitrust law, such as by requiring more providers to report any planned mergers, lowering the legal standards by which mergers are deemed anticompetitive, and mandating that providers receive approval from the government before merging. Another set of policies would increase the scope of antitrust law, such as by giving the FTC full authority to regulate nonprofit providers and outlawing certain anticompetitive contracting clauses. A third set of proposals would improve the infrastructure of antitrust enforcement, such as by increasing funding for antitrust agencies, creating agencies to monitor health care markets (as some states have done), and establishing specialized courts for antitrust cases.
Discussion
The FTC, DOJ, and states seek to promote competition in health care markets to encourage providers to lower costs for consumers and provide high quality medical care. Over the years, FTC, DOJ, and some states have challenged mergers as well as other anticompetitive practices. Nonetheless, there are inherent challenges to an approach that relies solely on efforts to foster competitive provider markets through antitrust regulation, particularly given the already high level of market concentration of providers across the country.
Several policy ideas have been floated at the federal and state level that are intended to strengthen antitrust regulation. However, given the challenges facing antitrust regulation and pro-competition policies, some policymakers have proposed a more direct regulatory approach, such as by capping prices or price growth or by establishing global budgets for hospitals. Some proponents of these approaches have highlighted that antitrust efforts and regulatory approaches could play complimentary roles. For instance, caps on health care prices could serve as a backstop in concentrated markets where at least some providers would not otherwise offer competitive rates or in small markets that are unable to support competition. Antitrust regulation may also play a useful role under price regulation, for example, by encouraging providers to compete for patients by offering higher quality care.
This work was supported in part by Arnold Ventures. KFF maintains full editorial control over all of its policy analysis, polling, and journalism activities.