This Week in Coronavirus: May 14 to 21
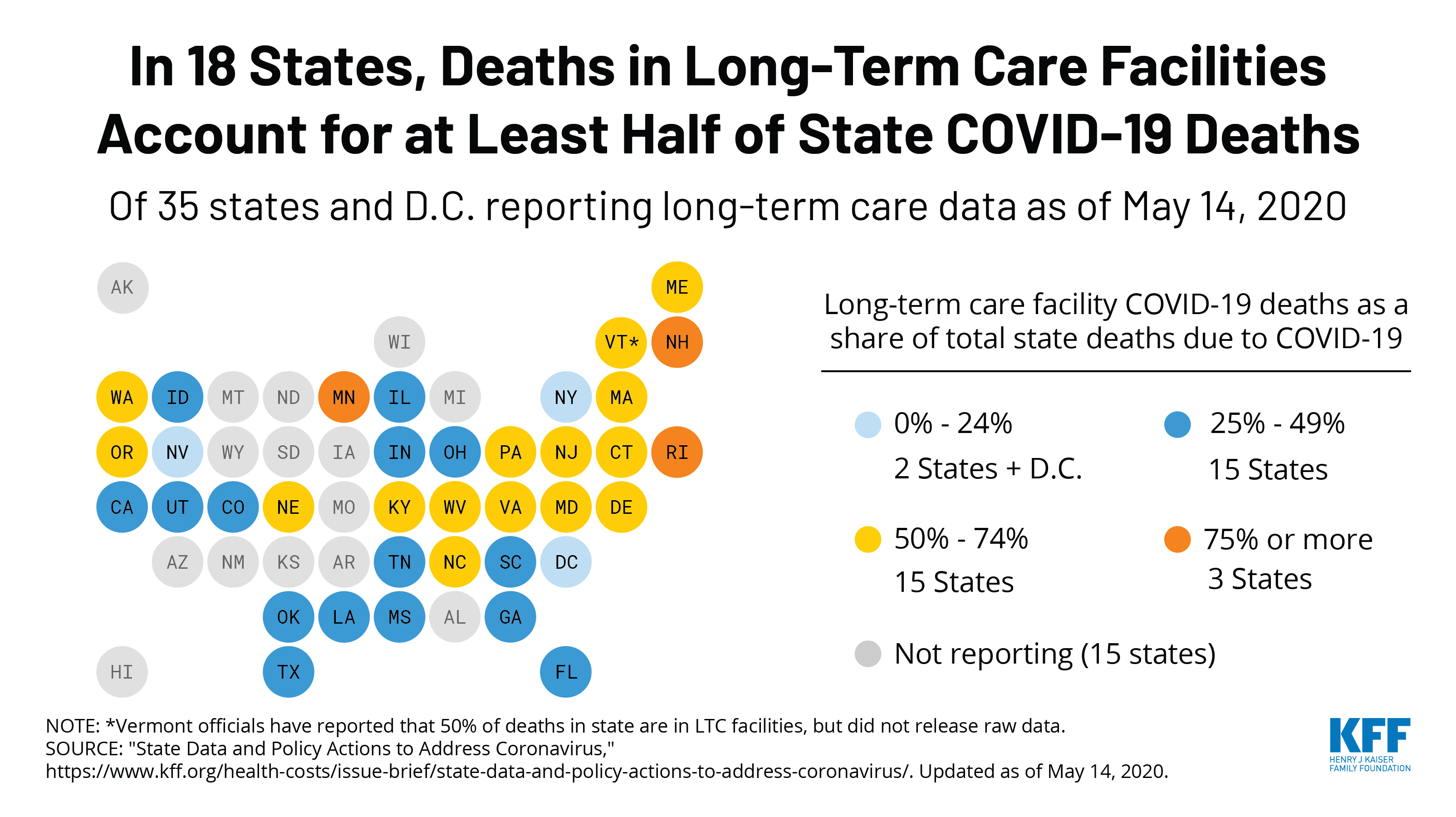
Every Friday, we’re recapping the latest on the coronavirus from our tracking, policy analysis, polling, and journalism. Total cases in the U.S. are still climbing, and this week increased by 159,400, bringing the cumulative total of cases past 1.5 million. This week, approximately 8,880 people died from COVID-19 in the U.S., bringing the total to 94,500. Across 38 states reporting this data yesterday, 42% of deaths occurred in long-term care facilities.
Meanwhile, since last Thursday, 11 more states have eased at least one social distancing requirement. Restaurants have reopened to dine-in service in 8 more states, and 7 states have eased or lifted their gatherings ban.
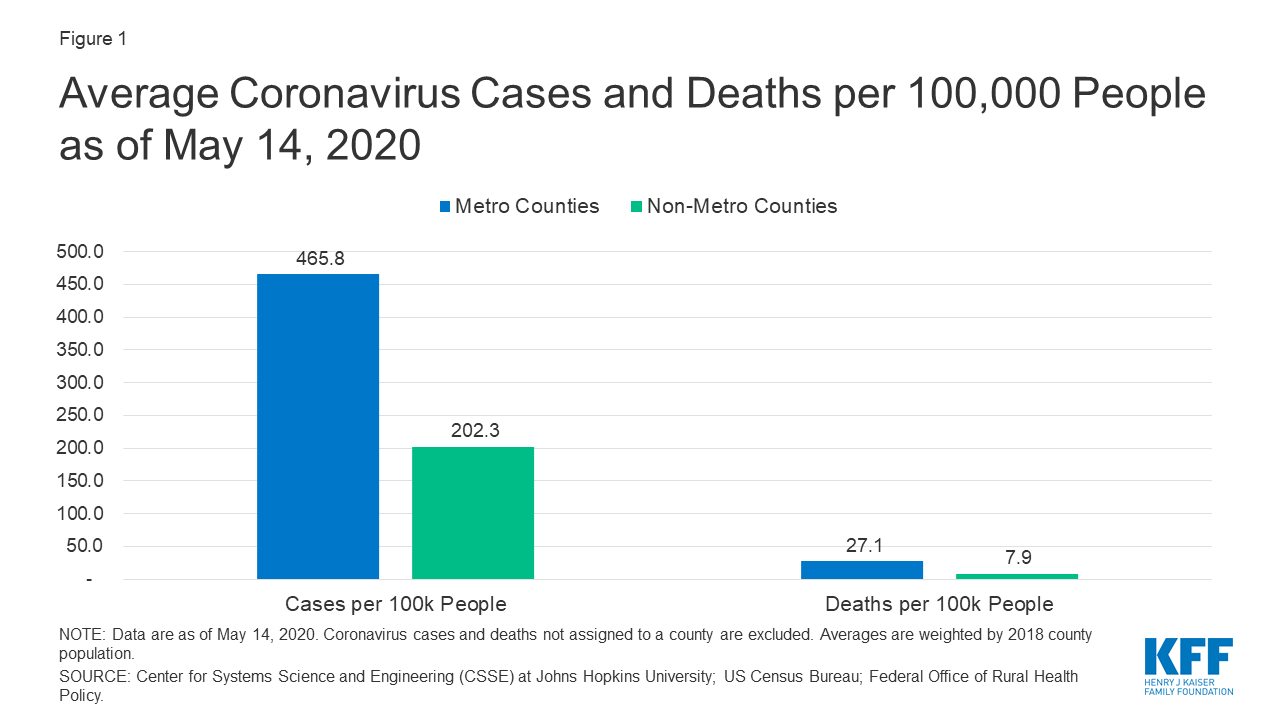
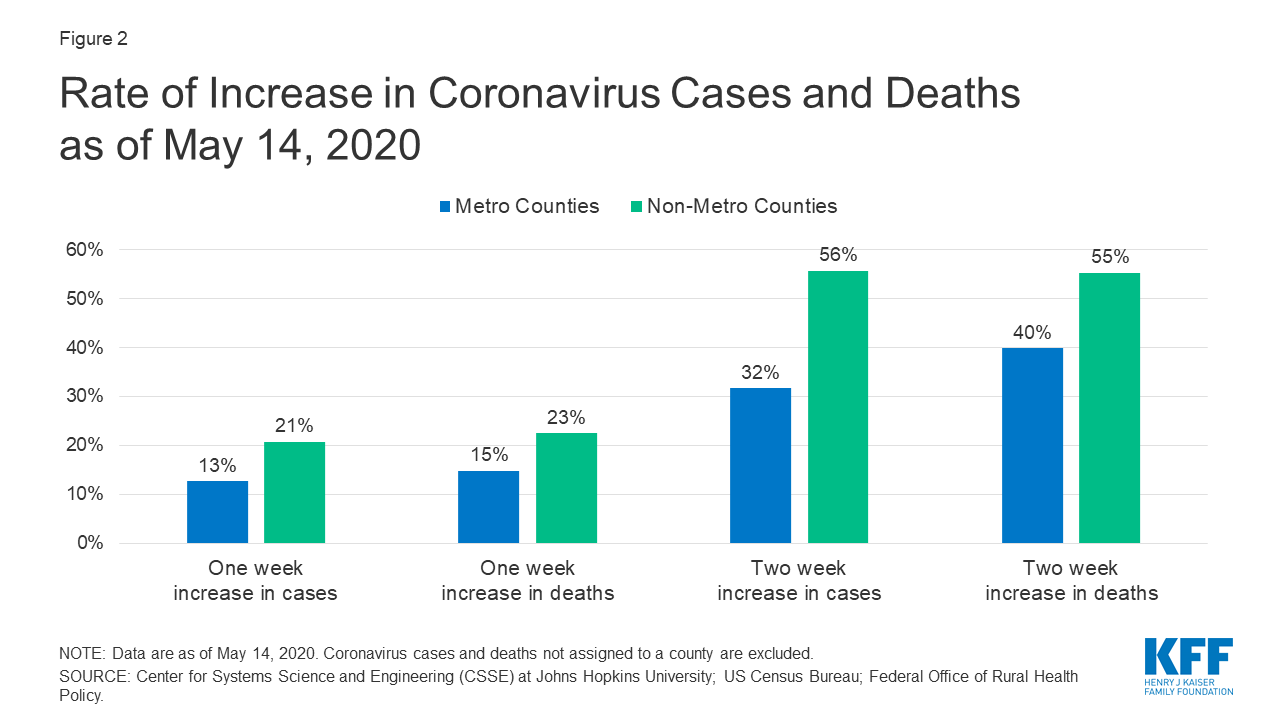
Recent KFF analysis finds that while non-metro areas currently have fewer coronavirus cases per capita, both cases and deaths are growing at a faster rate compared to metro counties. For example, non-metro counties saw a 56% increase in cases over a two week period, versus 32% in metro counties in the same period.
Our global COVID-19 Coronavirus Tracker has a new tab displaying cases/deaths by country on a timeline along with the actions world governments have taken to address the pandemic – such as closures of businesses and public services, school closures, and international travel restrictions. The United States timeline only shows federal actions. State-level policy actions can be found in our State Data and Policy Actions to Address Coronavirus tracker.
Here are more of the latest coronavirus stats from KFF’s tracking resources:
Global Cases and Deaths: This week, total cases worldwide passed 5 million – with approximately 660,300 new confirmed cases added between May 14 and May 21. There were approximately 30,500 new confirmed deaths worldwide between May 14 and May 21.
U.S. Cases and Deaths: There have been over 1.5 million total confirmed cases in the U.S. There were approximately 159,400 new confirmed cases and 8,880 confirmed deaths in the United States between May 14 and May 21.
U.S. Tests: There have been over 13 million total COVID-19 tests with results in the United States — with approximately 3 million added since May 14. 12% of the total tests were positive. There have been 39.8 tests with results per 1,000 people in the U.S.
State Reports of Long-Term Care Facility Cases and Deaths Related to COVID-19 (Includes Washington D.C.)
- Data Reporting Status: 47 states are reporting COVID-19 data in long-term care facilities, 4 states are not reporting
- Long-term care facilities with known cases: 7,732 (in 43 states)
- Cases in long-term care facilities: 174,381 (in 42 states)
- Deaths in long-term care facilities: 35,118 (in 37 states)
- Long-term care facilities as a share of total state cases: 16% (across 42 states)
- Long-term care facility deaths as a share of total state deaths: 42% (across 38 states)
State Social Distancing Actions (includes Washington D.C.):
- Social Distancing: 48 states have eased at least one social distancing measure.
- Stay At Home Order: Original stay at home order in place in 20 states, stay at home order eased or lifted in 25 states, no action in 6 states
- Mandatory Quarantine for Travelers: Original traveler quarantine mandate in place in 16 states, traveler quarantine mandate eased or lifted in 8 states, no action in 27 states
- Non-Essential Business Closures: Original non-essential business closures still in place in 3 states, some or all non-essential business permitted to reopen (some with reduced capacity) in 42 states, no action in 6 states
- Large Gatherings Ban: Original gathering ban/limit in place in 32 states, gathering/ban limit eased or lifted in 18 states, no action in 1 states
- State-Mandated School Closures: Closed in 7 states, closed for school year in 36 states, recommended closure in 1 state, recommended closure for school year in 6 states, rescinded in 1 state
- Restaurant Limits: Original restaurant closures still in place in 22 states, restaurants re-opened to dine-in service in 28 states, no action in 1 state
- Primary Election Postponement: Postponement in 14 states, cancelled in 1 state, no postponement in 36 states
- Emergency Declaration: There are emergency declarations in all states and D.C.
State COVID-19 Health Policy Actions (Includes Washington D.C.)
- Waive Cost Sharing for COVID-19 Treatment: 3 states require, state-insurer agreement in 3 states; no action in 45 states
- Free Cost Vaccine When Available: 9 states require, state-insurer agreement in 1 state, no action in 41 states
- States Requires Waiver of Prior Authorization Requirements: For COVID-19 testing only in 5 states, for COVID-19 testing and treatment in 6 states, no action in 40 states
- Early Prescription Refills: State requires in 18 states, no action in 33 states
- Premium Payment Grace Period: Grace period extended for all policies in 11 states, grace period extended for COVID-19 diagnosis/impacts only in 5 states, no action in 35 states
- Marketplace Special Enrollment Period: Marketplace special enrollment period in 12 states, no special enrollment period in 39 states
- Paid Sick Leave: 13 states enacted, 2 proposed, no action in 36 states
State Actions on Telehealth (Includes Washington D.C.)
38 states overall have taken mandatory action expanding access to telehealth services through private insurers, including:
- New Requirements for Coverage of Telehealth Services: Parity with in-person services in 6 states, broad coverage of telehealth services in 6 states, limited coverage of telehealth services in 6 states, no action in 33 states
- Waiving or Limiting Cost-Sharing for Telehealth Services: Waived for COVID-19 services only in 7 states, waived or limited for all services in 9 states, no action in 35 states
- Reimbursement Parity for Telehealth and In-Person Services: Required for all services in 17 states, no action in 34 states
- Require Expanded Options for Delivery of Telehealth Services: Yes in 35 states, for behavioral health services only in 1 state, no action in 15 states
Approved Medicaid State Actions to Address COVID-19 (Includes Washington D.C.)
- Approved Section 1115 Waivers to Address COVID-19: 1 state (Washington) has an approved waiver
- Approved Section 1135 Waivers: 51 states have approved waivers
- Approved 1915 (c) Appendix K Waivers: 43 states have approved waivers
- Approved State Plan Amendments (SPAs): 34 states have temporary changes approved under Medicaid or CHIP disaster relief SPAs, 1 state has an approved traditional SPA
- Other State-Reported Medicaid Administrative Actions: 51 states report taking other administrative actions in their Medicaid programs to address COVID-19
Adults at Higher Risk of Serious Illness if Infected with Coronavirus: 38% of all U.S. adults are at risk of serious illness if infected with coronavirus (92,560,223 total) due to their age (65 and over) or pre-existing medical condition. Of those at higher risk, 45% are at increased risk of serious illness if infected with coronavirus due to their existing medical condition such as such as heart disease, diabetes, lung disease, uncontrolled asthma or obesity. Among nonelderly adults — low-income, American Indian/Alaska Native & Black adults have a higher risk of serious illness if infected with coronavirus. In both cases – for race and household income – the higher risk of serious illness if infected with coronavirus is chiefly due to a higher prevalence of underlying health conditions and longstanding disparities in health care and other socio-economic factors.
This week’s posts in Coronavirus Policy Watch:
- Drew Altman on Big Questions for the Health Policy Community Emerging From the Coronavirus Crisis (Post)
The latest KFF COVID-19 resources:
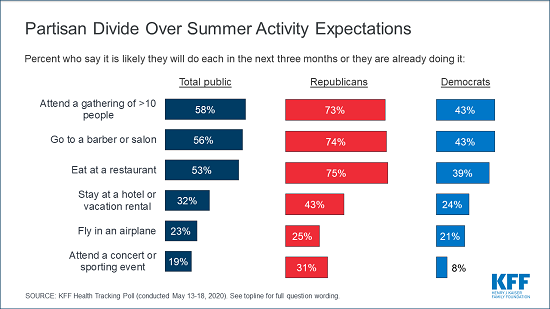
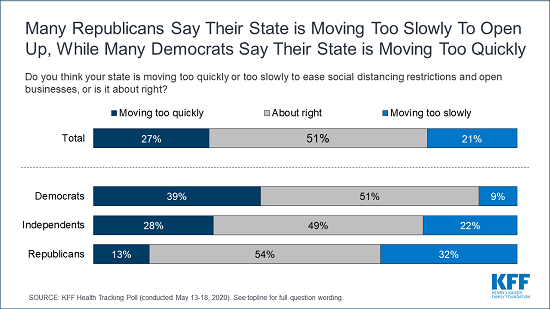
- KFF Health Tracking Poll – May 2020 (Poll Finding)
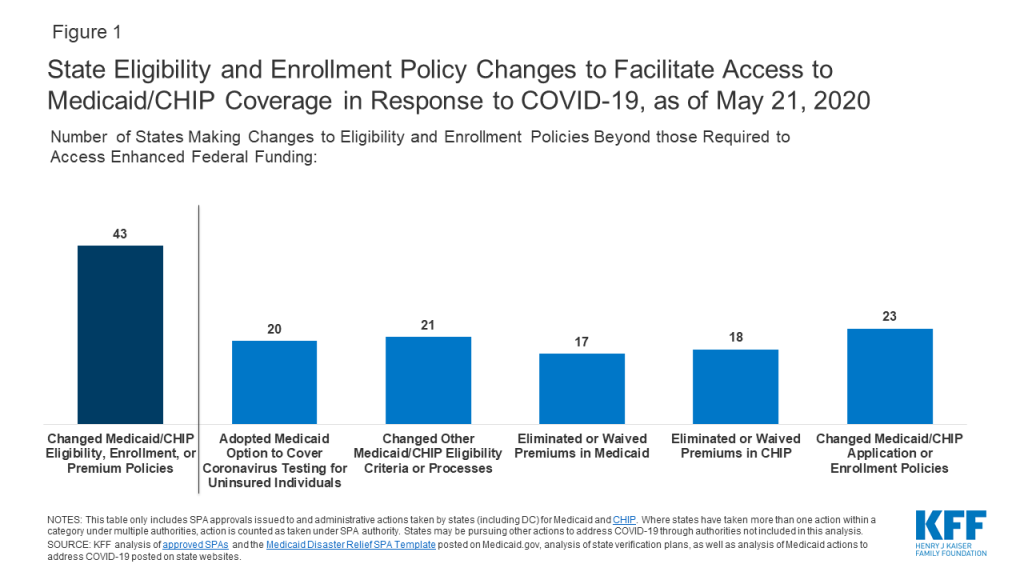
- State Actions to Facilitate Access to Medicaid and CHIP Coverage in Response to COVID-19 (Issue Brief)
- COVID-19 in Metropolitan and Non-Metropolitan Counties (Slideshow)
- Drew Altman: The Coronavirus Economy Could Make a Medicare Buy-In More Popular (Axios Column)
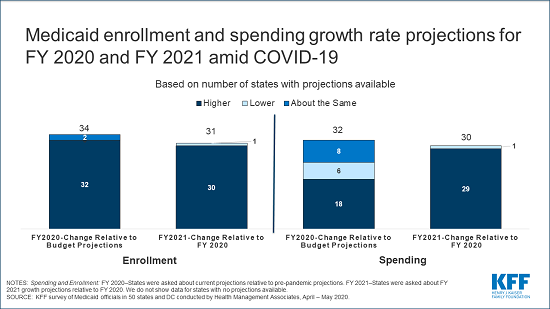
- How Have Healthcare Utilization and Spending Changed So Far During the Coronavirus Pandemic? (Chart Collection)
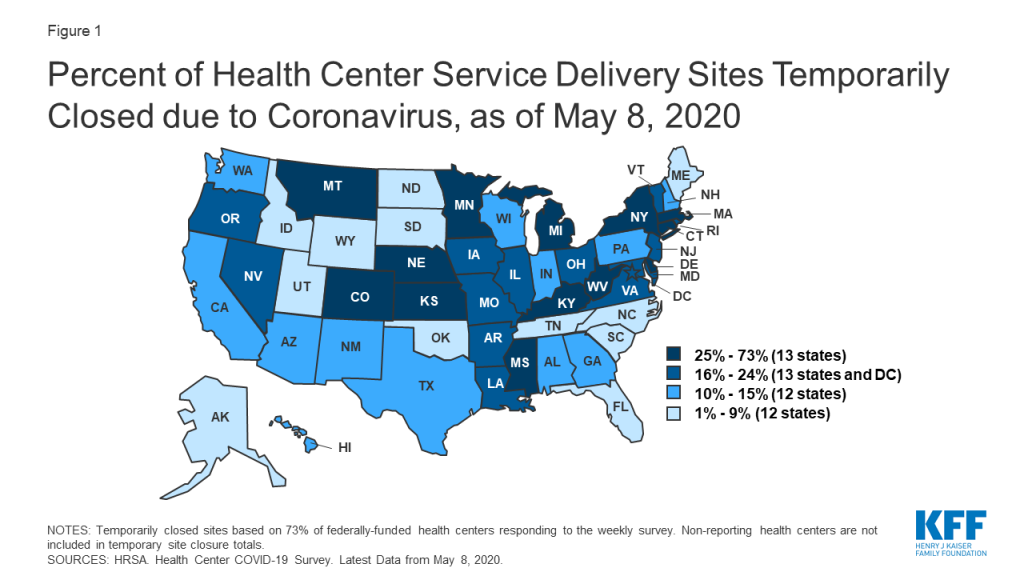
- Impact of Coronavirus on Community Health Centers (Issue Brief)
- COVID-19 & PEPFAR: Implications for the Future (Issue Brief)
- Updated: State Action to Limit Abortion Access During the COVID-19 Pandemic (Issue Brief)
- COVID-19 Pandemic Threatens To Push Millions Of People Into Poverty, U.N. SG, World Bank President Warn; Response Requires Coordination To Keep SDGs On Track, Guterres Says (KFF Daily Global Health Policy Report)
Trackers and Tools
- COVID-19 Coronavirus Tracker – Updated as of May 22
- State Data and Policy Actions to Address Coronavirus — Updated as of May 12
- Medicaid Emergency Authority Tracker: Approved State Actions to Address COVID-19 — Updated as of May 21
The latest KHN COVID-19 stories:
- Reopening Dental Offices For Routine Care Amid Pandemic Touches A Nerve (KHN, NBC)
- Scientist Has ‘Invisible Enemy’ In Sights With Microscopic Portraits Of Coronavirus (KHN, Time)
- Analysis: Get Ready For The Vaccine — They’re Never Simple (KHN, LA Times)
- KHN’s ‘What The Health?’: When It Comes To COVID-19, States Are On Their Own (Podcast)
- Congress Said COVID-19 Tests Should Be Free — But Who’s Paying? (KHN, NPR)
- The 30-Somethings Who Fled Big Cities To Shelter With Mom And Dad (KHN)
- Coronavirus Tests The Value Of Artificial Intelligence In Medicine (KHN, LA Times)
- Going The Distance By Bus Through A Pandemic (KHN, US News)
- Medicaid Providers At The End Of The Line For Federal COVID Funding (KHN, NPR)
- Tennessee’s Secret To Plentiful Coronavirus Testing? Picking Up The Tab (KHN, NPR)
- To Stem COVID, This Small Indiana City Decided To Test All Public-Facing Employees (KHN, Side Effects Public Media)
- Lost On The Frontline (KHN, The Guardian)
- ‘Last Responders’ Seek To Expand Postmortem COVID Testing In Unexplained Deaths (KHN, NPR)
- Drugmakers Tout COVID-19 Vaccines To Refurbish Their Public Image (KHN, Daily Beast)
- The Pandemic Is Hurting Pediatric Hospitals, Too (KHN, San Francisco Chronicle)
- How A Company Misappropriated Native American Culture To Sell Health Insurance (KHN, Fortune)
- Fewer Traffic Collisions During Shutdown Means Longer Waits For Organ Donations (KHN, KQED)
- Tourists, Beware: Foreign Visitors’ Travel Health Insurance Might Exclude Pandemics (KHN, The Guardian)
- ‘An Arm And A Leg’: Angst And Advice From A Health Insurance Insider (KHN)
- In The COVID Age, Bring A Mask And Gloves To A Protest (KHN)
- Listen: Tough Talk On Capitol Hill (KHN)