Update on the Status of Medication Abortion and the Courts
Laurie Sobel, Alina Salganicoff, and Mabel Felix
Published:
On April 21st, the US Supreme Court blocked a lower court order that would have stopped the distribution and availability of the medication abortion drug, mifepristone, across the country. The high court’s ruling allows the current FDA rules to remain in effect, keeping mifepristone available for medication abortion where and when abortion is legal as the case proceeds through the courts. Telehealth abortions can also continue, where state law permits.
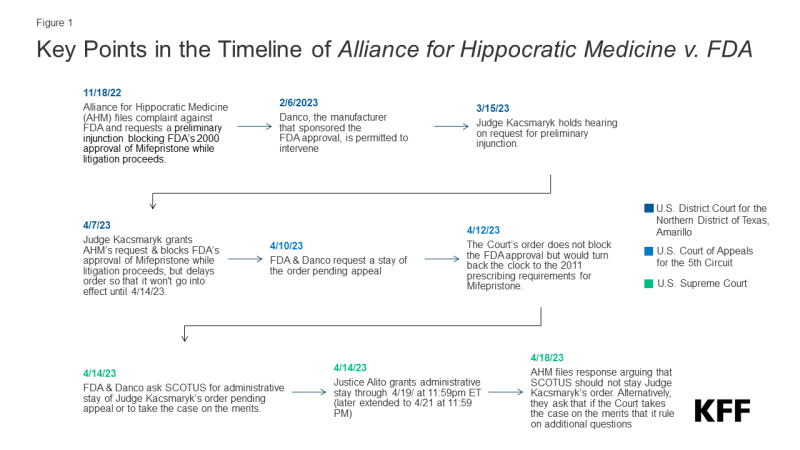
The court is responding to a ruling issued by Judge Matthew Kacsmaryk, the only judge in the US District Court for the Northern District of Texas Amarillo Division, who issued a preliminary injunction in the case, Alliance for Hippocratic Medicine v. FDA. The plaintiffs are challenging the FDA’s approval of mifepristone, one of the drugs used in medication abortion, claiming the FDA’s approval process and subsequent modifications of the conditions for dispensing mifepristone (known as REMS) as being beyond the FDA’s authority. The plaintiffs also contend that an 1873 anti-obscenity law, the Comstock Act, prohibits the mailing of any medication used for abortion (for details on the case see: Legal Challenges to the FDA Approval of Medication Abortion Pills).
Judge Kacsmaryk’s ruling would have blocked the FDA’s approval of mifepristone, which dates back to the year 2000. The judge delayed the enforcement of his own decision for seven days to give the FDA time to seek emergency relief from the U.S. Court of Appeals for the Fifth Circuit. The FDA and Danco Laboratories LLC (a party in the litigation, and the holder of the new drug application for Mifeprex, the brand name for mifepristone) appealed to Fifth Circuit Court of Appeals requesting a stay of Judge Kacsmaryk’s order pending the appeal (Figure 1). The Fifth Circuit Court of Appeals then issued an order which stayed part of the district court’s preliminary injunction (finding that the drug’s approval could not be revoked) but blocked the implementation of the changes that the FDA had made in 2016 to modify the agency’s conditions on the provision and dispensation of the drug (REMS). This decision would have effectively required the conditions that the FDA had in place 2011 to be re-introduced, taken the generic alternative drug off the market (GenBioPro), and required a relabeling of the drug. In addition, it would have required the drug to only be dispensed in person by a doctor, blocking the current ability of abortion providers and pharmacies to mail the drug.
On April 14, 2023, the FDA and Danco filed an emergency appeal with the Supreme Court, requesting either a stay of the district court’s ruling pending the appeal or for the Supreme Court to take the AHM case on an expedited basis. Later that day, Justice Alito, the justice assigned to emergency requests from the Fifth Circuit, issued an administrative stay keeping mifepristone available without any changes until April 19, 2023, which was subsequently extended to April 21, 2023.
On April 21, 2023, the Supreme Court blocked the district court’s ruling, sending the case back to the US Court of Appeals for the Fifth Circuit. Access to mifepristone will not change while this litigation continues through the final decision of the Supreme Court if their review is sought again.
It is important to recognize that this Supreme Court ruling is the not Court’s final word on the availability of mifepristone. The high court has sent this back to the Fifth Circuit to consider the appeal of the preliminary injunction (that is, the temporary block on mifepristone’s approval by Judge Kacsmaryk). The Fifth Circuit Court has scheduled an expedited hearing of the case for May 17, 2023, which will be the next step for this case. Procedurally, the Fifth Circuit is only considering the FDA and Danco’s appeal of the district court’s decision to temporarily block the FDA’s approval of mifepristone while the litigation proceeds. The district court has not yet considered the case on the merits. In the meantime, access to mifepristone will remain where abortion is permitted by state law.