Medicaid Moving Ahead in Uncertain Times: Results from a 50-State Medicaid Budget Survey for State Fiscal Years 2017 and 2018
Executive Summary
Medicaid covers one in five Americans, accounts for one in six dollars spent on health care in the United States and more than half of all spending for long-term services and supports, and is a state budget driver as well as the largest source of federal revenues to states. Medicaid is constantly evolving as policymakers strive to improve program value and outcomes through delivery system reforms, respond to economic conditions or public health concerns (such as the opioid epidemic), or implement federal policy changes including those in the Affordable Care Act (ACA) or other regulatory changes (like the recent Medicaid managed care rule). As states began state fiscal year (FY) 2018, Congress was debating major ACA repeal and replace legislation generating great uncertainty for states around Medicaid including the future of the ACA and financing for the Medicaid expansion as well as overall financing for the Medicaid program.
KFF #Medicaid budget survey has data on #opioids initiatives, provider rates, #managedcare & more
This report provides an in-depth examination of the changes taking place in Medicaid programs across the country during this time of uncertainty. The findings are drawn from the 17th annual budget survey of Medicaid officials in all 50 states and the District of Columbia conducted by the Kaiser Family Foundation (KFF) and Health Management Associates (HMA), in collaboration with the National Association of Medicaid Directors (NAMD). This report highlights certain policies in place in state Medicaid programs in FY 2017 and policy changes implemented or planned for FY 2018. The District of Columbia is counted as a state for the purposes of this report. Given differences in the financing structure of their programs, the U.S. territories were not included in this analysis.
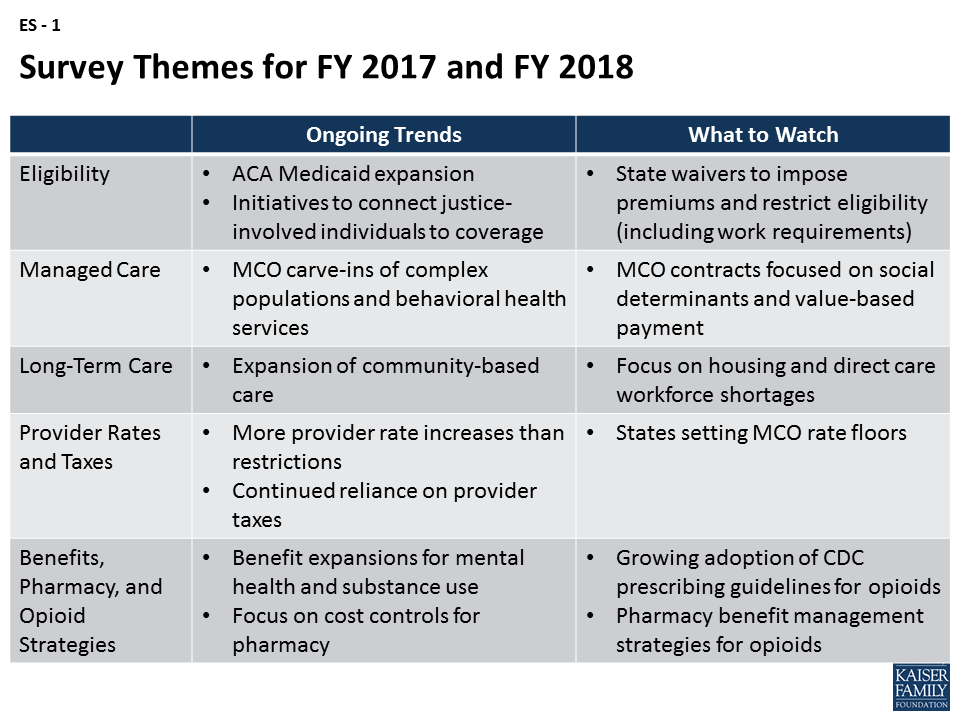
Key findings show that despite uncertainty about federal legislative changes, many states were continuing efforts to expand managed care, move ahead with payment and delivery system reforms, increase provider payment rates, and expand benefits as well as community-based long-term services and supports. Emerging trends include proposals to restrict eligibility (e.g., work requirements) and impose premiums through Section 1115 waivers, movement to include value-based purchasing requirements in MCO contracts, and efforts to combat the growing opioid epidemic. Key areas to watch include federal legislative efforts to restructure and limit federal Medicaid financing as well as Section 1115 waiver activity (state waiver proposals and CMS approvals). These issues will have implications for states, providers, and beneficiaries that could shape the future of the Medicaid program in FY 2018 and beyond (Figure ES – 1).
Eligibility Policies
Since 2014, most major eligibility changes have been related to adoption of the ACA Medicaid expansion. To date, 32 states have implemented the expansion (Louisiana was the latest state to adopt the expansion in FY 2017). Largely because the Medicaid expansion made many individuals involved in the criminal justice system newly eligible for coverage (including childless adults who were not previously eligible in most states), many states have implemented policies to facilitate enrollment in Medicaid upon release and to suspend, rather than terminate, Medicaid eligibility for incarcerated individuals. The majority of states also have policies in place to provide Medicaid coverage of inpatient care for those incarcerated in prisons or jails.
What to watch: Several non-expansion states (Idaho, Tennessee, Virginia, and Wyoming) reported this year that consideration of the Medicaid expansion was on hold due to uncertainty about the future of the Medicaid expansion option. For FY 2018, several states are seeking Medicaid eligibility restrictions through Section 1115 waivers, including conditioning eligibility on meeting work requirements,1 elimination of retroactive eligibility, and elimination of Medicaid expansion coverage for those with incomes above 100 percent of the federal poverty level (FPL).2 Eligibility provisions in proposals in Arkansas and Indiana would apply to ACA Medicaid expansion populations and proposals in Iowa, Maine, and Utah would apply to non-expansion populations. Two states (Arkansas and Indiana) reported activity related to Medicaid premiums in FY 2017 or FY 2018, both through Section 1115 waivers.
Managed Care And Delivery System Reforms
Managed care is the predominant delivery system for Medicaid in most states. Among the 39 states with comprehensive risk-based managed care organizations (MCOs), 29 states reported that 75 percent or more of their Medicaid beneficiaries were enrolled in MCOs as of July 1, 2017. More states continue to carve complex populations as well as behavioral health services into MCO contracts. Twenty-six of the 39 MCO states reported that they plan to use authority to receive federal matching funds for adults receiving inpatient psychiatric or substance use disorder (SUD) treatment in an institution for mental disease (IMD) for no more than 15 days a month included in the 2016 managed care regulations. Close to half of MCO states reported that the day limit is insufficient to meet acute inpatient or residential treatment needs for those with serious mental illness (SMI) or SUD.3 Nearly all states have managed care quality initiatives in place such as pay for performance or capitation withholds. Working in conjunction with or outside of MCO contracts, the majority of states (40) had one or more delivery system or payment reform initiative in place in FY 2017 (e.g., patient-centered medical home, ACA Health Home, accountable care organization, episode of care payment, or delivery system reform incentive program (DSRIP)).
What to watch: States are using MCO arrangements to increase attention to the social determinants of health and to promote value-based payment. States are increasingly requiring MCOs to: screen beneficiaries for social needs (19 states in FY 2017 and two additional states in FY 2018); provide care coordination pre-release to incarcerated individuals (six states in FY 2017 and one additional state in FY 2018); and use alternative payment models (APMs) to reimburse providers (13 states in FY 2017 set a target percentage of MCO provider payments that must be in APM and nine additional states plan to set a target in FY 2018). More than one in three states also have initiatives to expand dental access or improve oral health outcomes (for children and/or adults) and to expand the use of telehealth.
Long-Term Services And Supports (LTSS)
The vast majority of states in FY 2017 (47 states) and all states in FY 2018 are using a variety of tools and strategies to expand the number of people served in home and community-based settings. The most common strategies include using home and community-based services (HCBS) waivers or state plan options, serving more individuals through Programs of All-Inclusive Care for the Elderly (PACE), and building rebalancing incentives into managed long-term services and supports (MLTSS) contracts. Twenty-three states cover LTSS through one or more capitated managed care arrangements as of July 1, 2017.
What to watch: Housing supports are an increasingly important part of state LTSS benefits. Over half of states (27) reported that they implemented or expanded housing-related activities outlined in CMS’s June 2015 Informational Bulletin (e.g., housing transition services or housing and tenancy sustaining services) in FY 2017 or FY 2018 (up from 16 states reported last year). States are also focused on addressing LTSS direct care workforce shortages and turnover: 17 states reported efforts in FY 2017 or 2018 to increase wages for direct care workers and/or engage in targeted workforce development activities (recruiting, training, credentialing, etc.).
Provider Payment Rates And Taxes
In FY 2017 and FY 2018, more states made or are planning provider rate increases compared to restrictions across all provider types, except for inpatient hospital rates (hospital rate restrictions are primarily rate freezes, which are counted as restrictions in this report). All states except Alaska rely on provider taxes and fees to provide a portion of the non-federal share of the costs of Medicaid. Three states indicated plans for new provider taxes in FY 2018 and 13 states plan provider tax increases.
What to watch: Survey responses related to MCO rate setting show that 18 of 39 MCO states require MCO rates to follow fee-for-service (FFS) rate changes for some provider types, and two states require MCO rates to follow FFS rate changes for all provider types. Twenty-four states reported they had MCO rate floors for some provider types, and five states said they had rate floors for all types of Medicaid providers. Federal legislation considered in the Senate proposed limiting the use of provider taxes by lowering the “safe harbor threshold” from the current allowable level, 6.0 percent of net patient revenues, to 5.0 percent of net patient revenues by FY 2025 in one proposal and 4.0 percent by FY 2025 in another. The survey shows that 29 states reported having at least one provider tax exceeding 5.5 percent of net patient revenues and 46 states reported having at least one provider tax exceeding 3.5 percent as of July 1, 2017.
Benefits, Prescription Drugs, and Opioid Strategies
A total of 26 states expanded or enhanced covered benefits in FY 2017 and 17 states plan to add or enhance benefits in FY 2018, most commonly for behavioral health/substance use disorder services and dental services. Thirteen states reported changes to copayment requirements in either FY 2017 or FY 2018, including new or increased copayments for enrollees with income above 100 percent FPL, for non-emergency use of a hospital emergency department, and pharmacy. Most states identified high cost and specialty drugs (including hepatitis C antivirals) as a significant cost driver for state Medicaid programs. The majority reported actions to refine and enhance their pharmacy programs, especially implementation of new utilization controls (e.g., prior authorization requirements, clinical edits, and quantity limits). Thirty-five of 39 MCO states reported that the pharmacy benefit was “generally carved-in.” Of these 35 states, the majority reported requirements that MCOs have uniform clinical protocols (31 states) or uniform preferred drug lists (PDLs) (19 states) that will be in place for one or more drugs as of the end of FY 2018.
What to watch: A growing number of states have chosen to adopt the CDC guidelines for the prescribing of opioid pain medications for adults in primary care settings (34 states as of the end of FY 2018). Nearly all states have various FFS pharmacy management strategies targeted at opioid harm reduction in place as of FY 2017, including quantity limits (48 states); clinical criteria claim system edits (46 states); step therapy (34 states); and other prior authorization requirements (32 states). Somewhat fewer states (28 states) reported requirements in place for Medicaid prescribers to check their states’ Prescription Drug Monitoring Program before prescribing opioids to a Medicaid patient. Among the 35 states that used MCOs to deliver pharmacy benefits, 24 reported that they required MCOs to follow some or all of their FFS pharmacy benefit management policies for opioids. For FY 2017, the vast majority of states (46 states) reported that naloxone (a prescription opioid overdose antidote) was available in at least one formulation without prior authorization (PA) and most states (42) also covered the naloxone nasal spray formulation without PA. The standard of care for opioid use disorder is medication-assisted treatment (MAT), which combines psychosocial treatment with medication. All 49 states that responded to a new question about medication-assisted treatment (MAT) drugs reported coverage of buprenorphine and both oral and injectable naltrexone, but a somewhat smaller number (36 states) reported coverage of methadone in FY 2017.4
Looking Ahead
Medicaid is constantly evolving as policymakers strive to improve program value and outcomes through delivery system reforms, respond to economic conditions or public health concerns (such as the opioid epidemic), or implement federal policy changes including those in the ACA or other regulatory changes (like the recent Medicaid managed care rule). As states began FY 2018, Congress was debating major ACA repeal and replace legislation generating great uncertainty for states around Medicaid including the future of the ACA and financing for the Medicaid expansion as well as overall financing for the overall Medicaid program. On this year’s survey, Medicaid directors were asked to comment on state-specific implications of federal proposals. Most Medicaid directors from the 32 ACA Medicaid expansion states reported that they would not be able to continue covering the expansion population, or that coverage would be at substantial risk, if the ACA enhanced federal match for this population were terminated. Almost all Medicaid directors expressed concern about the likely negative fiscal consequences tied to proposed limits on federal Medicaid spending. Some directors mentioned that they welcomed potential new state policy flexibility under federal legislative proposals, but a greater number of Medicaid directors expressed concern that proposals to convert Medicaid to a per capita cap or block grant would not provide sufficient flexibility to enable states to make up for the reduction in federal funds.
Despite the uncertain policy environment, many states continue efforts to expand managed care, move ahead with payment and delivery system reforms, increase provider payment rates, expand benefits, and expand community-based LTSS. Emerging trends from this year’s survey include proposals to restrict eligibility (e.g., work requirements) and impose premiums through Section 1115 waivers, movement to include value-based purchasing requirements in MCO contracts, and efforts to combat the growing opioid epidemic. Key areas to watch include federal legislative efforts to restructure and limit federal Medicaid financing as well as Section 1115 waiver activity (state waiver proposals and CMS approvals). These issues will have implications for states, providers, and beneficiaries that could shape the future of the Medicaid program in FY 2018 and beyond.
Report: Introduction
Medicaid provides health insurance coverage to more than one in five Americans, and accounting for over one-sixth of all U.S. health care expenditures.5 The Medicaid program constantly evolves, as policy makers in each state make changes to improve their programs, respond to economic conditions, come into compliance with new federal requirements, and implement other state budget and policy priorities. As fiscal year (FY) 2018 began in most states, legislative proposals to repeal major portions of the Affordable Care Act (ACA), including the Marketplace and Medicaid coverage expansions, were under consideration in Congress. These proposals would also have fundamentally restructured federal Medicaid financing, converting the current open-ended entitlement to a federal block grant or per capita cap. It is within that context that this year’s survey was conducted.
This report examines the reforms, policy changes, and initiatives that occurred in FY 2017 and those adopted for implementation for FY 2018 (which began for most states on July 1, 20176 ). Report findings are drawn from the annual budget survey of Medicaid officials in all 50 states and the District of Columbia conducted by the Kaiser Family Foundation (KFF) and Health Management Associates (HMA), in collaboration with the National Association of Medicaid Directors (NAMD). This was the 17th annual survey, which has been conducted from FY 2002 through FY 2018. (Copies of previous reports are archived here.7 )
The KFF/HMA Medicaid survey on which this report is based was conducted from June through September 2017. The survey was sent to each state Medicaid director in June 2017. Directors and their staff provided data for this report in their written survey response and through a follow-up telephone interview. All 50 states and DC completed surveys and participated in telephone interview discussions between July and September 2017. Given differences in the financing structure of their programs, the U.S. territories were not included in this analysis. An acronym glossary and the survey instrument are included as appendices to this report.
The survey collects data about Medicaid policies in place or implemented in FY 2017, policy changes implemented at the beginning of FY 2018, or policy changes for which a definite decision has been made to implement in FY 2018. Some policies adopted for the upcoming year are occasionally delayed or not implemented for reasons related to legal, fiscal, administrative, systems or political considerations, or due to delays in approval from CMS. The District of Columbia is counted as a state for the purposes of this report; the counts of state policies or policy actions that are interspersed throughout this report include survey responses from the 51 “states” (including DC). Key findings of this survey, along with state-by-state tables providing more detailed information, are described in the following sections of this report:
Report: Eligibility And Premiums
Key Section Findings
Since 2014, most major eligibility changes have been related to adoption of the ACA Medicaid expansion. To date, 32 states have implemented the expansion (Louisiana was the latest state to adopt the expansion in FY 2017). Only a few states adopted other Medicaid eligibility expansions for FYs 2017 or 2018, and these changes were generally narrow in scope and targeted to a limited number of beneficiaries. The majority of states have policies in place to provide Medicaid coverage of inpatient care for those incarcerated in prisons or jails, to facilitate enrollment in Medicaid upon release, and to suspend, rather than terminate, Medicaid eligibility for incarcerated individuals.
What to watch:
- For FY 2018, several states are seeking Medicaid eligibility restrictions through Section 1115 waivers that apply to ACA Medicaid expansion and/or traditional Medicaid populations, including the addition of work requirements, elimination of retroactive eligibility, and elimination of Medicaid expansion coverage for those with income above 100 percent of the federal poverty level (FPL).8 Several non-expansion states reported that consideration of the Medicaid expansion was on hold due to uncertainty about the future of the Medicaid expansion option.
- Two states reported activity related to premiums in FY 2017 or FY 2018, both through Section 1115 waivers.
Tables 1, 2, and 3 at the end of this section include additional details on eligibility and premium policy changes in FYs 2017 and 2018.
Changes to Eligibility Standards
The ACA Medicaid expansion was one of the most significant Medicaid eligibility changes in the history of the program. By FY 2017, 32 states had implemented the ACA Medicaid expansion: 26 states in FY 2014; three states (Indiana, New Hampshire and Pennsylvania) in FY 2015; two states (Alaska and Montana) in FY 2016, and one state (Louisiana) on July 1, 2016 (FY 2017) (Figure 1).
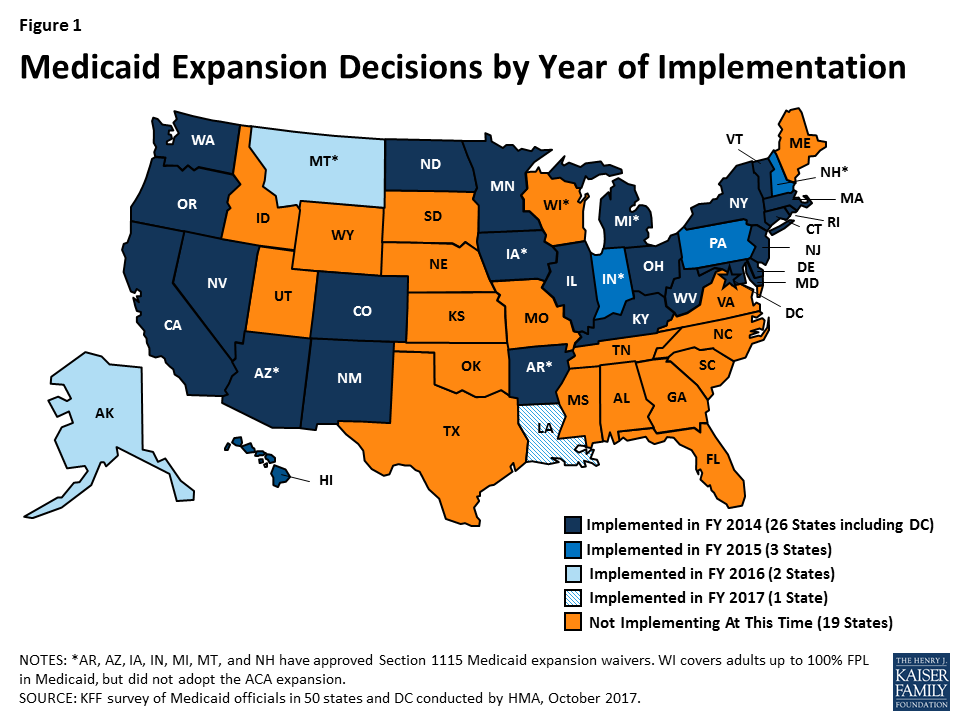
Several non-expansion states (Idaho, Tennessee, Virginia, and Wyoming) reported that consideration of the Medicaid expansion was on hold due to uncertainty about the future of the Medicaid expansion option. North Carolina’s governor announced plans to adopt the expansion shortly after taking office in January 2017. These plans have been delayed, however, by a lawsuit brought by a group of legislators challenging the governor’s authority to expand without legislative approval.9 In Maine, voters will decide whether the state will adopt the ACA Medicaid expansion in a referendum this November.10
Other Eligibility Expansions
Beyond the ACA Medicaid expansion, states have made very few changes to expand Medicaid eligibility since 2014, and states reported only a few narrow expansions targeting a limited number of beneficiaries implemented in FY 2017 or planned for FY 2018 (Tables 1 and 2).
In addition to the ACA Medicaid expansion in Louisiana, a total of six other states made changes that expanded Medicaid eligibility in FY 2017. For FY 2018, seven states plan to implement eligibility expansions. Notable expansions reported include the following:
- In FY 2017, both Florida and Utah implemented the option to eliminate the five-year bar on Medicaid eligibility for lawfully-residing immigrant children. Arkansas and Nevada both intend to implement this option in FY 2018 (pending CMS approval of their plans, which were adopted by both states’ legislatures during FY 2017).
- In FY 2018, a pending Section 1115 waiver in Utah proposes covering a new eligibility group: individuals with income below 5 percent of the FPL who are chronically homeless, justice-involved, or individuals in need of substance use and/or mental health treatment.
Eligibility Restrictions
Only one state reported implementing an eligibility restriction in FY 2017: Missouri suspended its family planning waiver11 in FY 2017 following legislative restrictions contained in the state’s FY 2017 appropriations bill.12 Although Missouri replaced the Medicaid family planning waiver with a state-funded family planning program, this change eliminated Medicaid coverage for family planning services and placed new restrictions on which providers are accessible to the population. (The new restrictions apply only to individuals eligible through the waiver, however, and do not affect coverage of family planning services for other Medicaid eligible individuals.)
Eight states reported eligibility restrictions for FY 2018 (six states through Section 1115 waivers and two states through state plan authority), some in response to a March 2017 Trump administration letter to state governors13 that signaled an openness to approve Section 1115 waivers that include work requirements and more expansive use of premiums and cost sharing. This year’s survey captured changes that states have implemented or plan to implement in FY 2018, even if these changes are included in Section 1115 Waiver proposals that are pending approval 14 at CMS. Waiver provisions (in approved or pending waivers) that states plan to implement in FY 2019 or after are described later in the “Challenges and Priorities” section of this report.15 A description of key eligibility restrictions included in pending Section 1115 waivers planned for FY 2018 implementation follows.
FY 2018 restrictions for ACA Medicaid expansion populations:16
- Arkansas 17 has proposed to amend its “Arkansas Works” Medicaid expansion waiver to: (1) eliminate coverage for persons with income above 100 percent of the FPL while still maintaining the enhanced federal matching rate for the remaining expansion population at or below 100 percent FPL, (2) include a work requirement for the remaining expansion population, and (3) eliminate the conditions CMS placed on the state’s waiver of retroactive eligibility for expansion enrollees (including the medically frail).18
- Indiana 19 plans to impose a three-month lock-out from coverage on individuals who fail to comply with redetermination requirements. Beneficiaries who fail to verify eligibility at renewal would be disenrolled but could re-enroll without a new application if they provide necessary documentation within 90 days. After 90 days, a three-month lock-out period would follow before individuals could re-enroll.20
FY 2018 restrictions for non-ACA expansion Medicaid populations:
- Iowa plans to eliminate retroactive Medicaid eligibility for all Medicaid enrollees with an October 1, 2017 target implementation date.21
- Maine 22 plans to: (1) waive retroactive eligibility so that coverage would begin no earlier than the first day of the month of application, (2) impose a work requirement for adults (ages 19 to 64), such as parents and former foster care youth, and a time limit on coverage for those who fail to comply with work requirement, (3) apply a $5,000 asset test to all coverage groups that currently do not have an asset test, and (4) eliminate hospital presumptive eligibility for all coverage groups. The state’s pending waiver application proposes to implement these initiatives within six months of demonstration approval (the state’s estimated start date is January 1, 2018).23
- Utah plans to impose a work requirement for its existing Primary Care Network (PCN) waiver adults,24 impose a 60-month time limit on eligibility for PCN adults, and end hospital presumptive eligibility for all current enrollees.
Utah’s Proposed Section 1115 Limited Medicaid Coverage Expansion
A pending Section 1115 waiver in Utah proposes covering a new eligibility group: individuals with income below 5 percent of the FPL who are chronically homeless, justice-involved, or individuals in need of substance use and/or mental health treatment. The state also plans to implement the following restrictive policies for this proposed new childless adults coverage group: 60-month time limit on coverage; no retroactive eligibility; and no hospital presumptive eligibility. Implementation is proposed for January 1, 2018.25
Premiums
The Medicaid statute generally does not allow states to charge premiums to Medicaid beneficiaries. Only two states reported activity related to Medicaid premiums in either FY 2017 or FY 2018.26 In FY 2017, Arkansas replaced the requirement that expansion enrollees make contributions to “Health Independence Accounts” with a new 2 percent of income premium requirement (up to $13/month) for expansion enrollees above 100 percent FPL. Indiana’s pending waiver includes requests to: (1) add a 1 percent premium surcharge for tobacco users beginning in the second year of enrollment, (2) require Transitional Medical Assistance parents with income up to 138 percent FPL to pay premiums like expansion adults, and (3) change to a tiered premium structure instead of a flat charge of 2 percent of income (this change is planned for FY 2018 and expected to have a neutral effect on beneficiaries) (Table 2).
Coverage Initiatives for the Criminal Justice Population
In recent years, many states have implemented new policies to connect individuals involved with the criminal justice system to Medicaid given that the Medicaid expansion made many of these individuals newly eligible for coverage (including childless adults who were not previously eligible in most states). Connecting these individuals to health coverage27 can facilitate their integration back into the community. Individuals may be enrolled in Medicaid while they are incarcerated, but Medicaid cannot cover the cost of their care during their period of incarceration, except for inpatient services. Nearly all states have policies in place to cover inpatient care for individuals who are incarcerated under Medicaid. Most states are also working with corrections agencies and with local jails to facilitate enrolling individuals in Medicaid before they are released. In addition, half of the states (25) have enrollment initiatives to facilitate Medicaid enrollment for parolees. Some states train criminal justice employees to assist with Medicaid applications and other states have dedicated Medicaid staff to work with the corrections agencies to facilitate enrollment for inmates or payment for inpatient care of inmates. Finally, the majority of states suspend rather than terminate Medicaid coverage for enrollees who become incarcerated. When coverage is suspended, it can be reinstated more easily and quickly upon release from incarceration or when an inpatient hospital stay occurs.28
While both Medicaid expansion and non-expansion states have adopted these strategies to connect justice-involved individuals to Medicaid coverage, these initiatives affect many more people in expansion states because eligibility for adults remains restrictive in non-expansion states. In this year’s survey, one non-expansion state commented that the administrative costs of implementing Medicaid coverage policies for the criminal justice population would be excessive since the policies would apply to such a small number of people in the state.
Details on Medicaid coverage for individuals involved with the criminal justice system are included in Exhibit 1 and Table 3.
| Exhibit 1: Coverage Initiatives for the Criminal Justice Population in FY 2017 and/or FY 2018 (# of States) | |||
| Select Medicaid Coverage Policies for the Criminal Justice Population | Jails | Prisons* | Parolees |
| Medicaid coverage for inpatient care provided to incarcerated individuals | 41 | 47 | N/A |
| Medicaid outreach/assistance strategies to facilitate enrollment prior to release from incarceration or for parolees | 33 | 40 | 25 |
| Eligibility suspended (rather than terminated) for Medicaid enrollees who become incarcerated^ | 36 | 37 | N/A |
| ^States that continue Medicaid eligibility for incarcerated individuals but limit covered benefits to inpatient hospitalization are also included in the count of states that suspend eligibility. *The District of Columbia has jails but not a prison system. However, DC is counted under Medicaid outreach/assistance strategies because some individuals who serve prison terms outside of DC may be placed in residential re-entry centers upon returning to DC and may apply for Medicaid to access coverage for 24-hour inpatient care and to facilitate enrollment prior to release. | |||
Louisiana Medicaid and Corrections Policies
Louisiana has implemented several strategies to increase coverage and access to care for individuals released from incarceration, particularly those with high health care needs.Louisiana Medicaid shares data with the Louisiana Department of Corrections (LDOC), which adds incarceration and release dates to the Medicaid eligibility system. As a result of this data sharing, the state Medicaid agency can automatically identify individuals pre-release and begin planning nine months before the scheduled release date. Additionally, in FY 2017 the state began using a new system and streamlined application to enroll state prisoners in Medicaid prior to release and connect them to a health plan. As part of this process, the system also identifies high need individuals for discharge planning/case management. There are “high needs” markers for those with serious mental illness, substance use disorder, co-morbid medical conditions, or those who are “bed bound”. The Medicaid health plans are required to do pre-release care planning and ensure that there will be sufficient medications available at discharge for these high-need individuals.
Plans are underway to expand outreach/ enrollment assistance to local jails in FY 2018.
Table 1: Changes to Eligibility Standards in all 50 States and DC, FY 2017 and FY 2018
| Eligibility Standard Changes | ||||||
| States | FY 2017 | FY 2018 | ||||
| (+) | (-) | (#) | (+) | (-) | (#) | |
| Alabama | ||||||
| Alaska | ||||||
| Arizona | ||||||
| Arkansas | X | X | X | |||
| California | ||||||
| Colorado | X | X | ||||
| Connecticut | ||||||
| Delaware | ||||||
| DC | ||||||
| Florida | X | |||||
| Georgia | ||||||
| Hawaii | ||||||
| Idaho | X | |||||
| Illinois | ||||||
| Indiana | X | X | ||||
| Iowa | X | |||||
| Kansas | ||||||
| Kentucky | ||||||
| Louisiana | X-Medicaid Expansion | |||||
| Maine | X | X | ||||
| Maryland | ||||||
| Massachusetts | X | |||||
| Michigan | ||||||
| Minnesota | X | |||||
| Mississippi | ||||||
| Missouri | X | X | ||||
| Montana | ||||||
| Nebraska | ||||||
| Nevada | X | |||||
| New Hampshire | ||||||
| New Jersey | ||||||
| New Mexico | X | |||||
| New York | ||||||
| North Carolina | ||||||
| North Dakota | ||||||
| Ohio | X | |||||
| Oklahoma | ||||||
| Oregon | ||||||
| Pennsylvania | ||||||
| Rhode Island | ||||||
| South Carolina | ||||||
| South Dakota | ||||||
| Tennessee | ||||||
| Texas | ||||||
| Utah | X | X | X | |||
| Vermont | ||||||
| Virginia | X | X | ||||
| Washington | ||||||
| West Virginia | ||||||
| Wisconsin | ||||||
| Wyoming | X | |||||
| Totals | 7 | 1 | 1 | 7 | 8 | 2 |
| NOTES: From the beneficiary’s perspective, positive changes counted in this report are denoted with (+), negative changes are denoted with (-), and neutral changes are denoted with (#). This table captures eligibility changes that states have implemented or plan to implement in FY 2017 or 2018, including changes that are part of pending Section 1115 waivers. For pending waivers, only provisions planned for implementation before the end of FY 2018 (according to waiver application documents) are counted in this table. Waiver provisions in pending waivers that states plan to implement in FY 2019 or after are not counted here.SOURCE: Kaiser Family Foundation Survey of Medicaid Officials in 50 states and DC conducted by Health Management Associates, October 2017. | ||||||
Table 2: States Reporting Eligibility29 and Premium30 Changes in FY 2017 and FY 201831
| State | Fiscal Year | Eligibility Changes |
| Arkansas | 2017 | Premiums (New only for expansion population, under Sec. 1115 waiver): Arkansas Works program ended prior required contributions to “Health Independence Accounts” and replaced them with a 2% premium requirement for expansion populations with income 100-133% FPL (up to $13/month). Non-payment does not affect eligibility, but a debt to the state is accumulated (1/1/2017). |
| 2018 | Expansion Adults (-) Pending Sec. 1115 Waiver: Eliminate the conditions CMS placed on the state’s waiver of retroactive eligibility for expansion enrollees (including the medically frail), effective 1/1/2018 (60,000 individuals).32 Expansion Adults (-) Pending Sec. 1115 Waiver: Eliminate coverage for expansion population with income 100-133% FPL. (Implementation phased based on redetermination date.) Expansion Adults (-) Pending Sec. 1115 Waiver: Work requirement for “remaining” expansion adults (0-100% FPL), similar to SNAP program. Expansion Adults (#) Pending Sec. 1115 Waiver: End premium assistance program for employer sponsored insurance (40 individuals). Children (+): Implement the CHIPRA option to eliminate the 5-year bar on Medicaid eligibility for legally-residing immigrant children. | |
| Colorado | 2017 | Adults (+): Implementing annualized income for eligibility for MAGI populations (affects 3,000). |
| 2018 | Aged & Disabled (+) Planned Sec. 1115 Waiver: Medicaid buy-in option for individuals in support living services, spinal cord injury, & brain injury waivers. | |
| Florida | 2017 | Children (+): Implement the CHIPRA option to eliminate the 5-year bar on Medicaid eligibility for legally-residing immigrant children. |
| Idaho | 2018 | Children (+): Cover children with severe emotional disorder in families with income between 185 and 300% FPL (1,000 children). |
| Indiana | 2018 | Expansion Adults (-) Pending Sec. 1115 Waiver: Three-month lock-out of coverage following a 90-day period of disenrollment for failure to comply with redetermination requirements. Expansion Adults (#): End HIP Link premium assistance program for Employer Sponsored Insurance. (Enrollees will be moved to other HIP 2.0 coverage). Premiums (New) Pending Sec. 1115 Waiver: Require Transitional Medical Assistance parents up to 138% FPL to pay premiums like expansion adults. Premiums (New) Pending Sec. 1115 Waiver: Add a 1% premium surcharge for tobacco users beginning in the second year of enrollment. Premiums (Neutral for Expansion Population) Pending Sec. 1115 Waiver: Seeking a tiered contribution amount instead of flat 2% of income, effective February 1, 2018 for the HIP 2.0 program. |
| Iowa | 2018 | All Groups (-) Pending Sec. 1115 Waiver: Eliminate retroactive eligibility, target effective date 10/1/17. |
| Louisiana | 2017 | Expansion Adults (+): Implemented Medicaid expansion on July 1, 2016 (430,000 individuals). |
| Maine | 2017 | Adults (+): Increased eligibility under family planning pathway to 209% FPL. |
| 2018 | Adults (-) Pending Sec. 1115 Waiver: Add a work requirement for many groups of adults ages 19-64: parents, former foster care youth, individuals receiving transitional medical assistance, medically needy parents/caretakers, individuals eligible for family planning services only, and individuals with HIV. Those who fail to comply with work requirement would be limited to no more than 3 months in a 36-month period. All Groups (-) Pending Sec. 1115 Waiver: Eliminate retroactive eligibility. Adults (-) Pending Sec. 1115 Waiver: Apply a $5,000 asset test to all coverage groups that do not currently have an asset test (under current law there is no asset test for coverage groups based solely on low income (vs. old age/disability)). All Groups (-) Pending Sec. 1115 Waiver: Eliminate hospital presumptive eligibility. | |
| Massachusetts | 2018 | Adults (-) Pending Sec. 1115 Waiver: Eliminate 90 day period of provisional eligibility for adults under age 65 without verified income who are not either pregnant or HIV positive (130,000).33 |
| Minnesota | 2017 | Aged & Disabled (+): Increased income standard for the medically needy from 75% FPL to 80% FPL on 7/1/2016. Adults (+): Added optional Medicaid eligibility group for family planning for those with income up to 278% FPL. |
| Missouri | 2017 | Adults (-): Family Planning Waiver ended and replaced with a state-only (non-Medicaid) program. |
| 2018 | Aged & Disabled (+): Asset limit doubled (10,005 individuals). | |
| Nevada | 2018 | Children (+): Implement the CHIPRA option to eliminate the 5-year bar on Medicaid eligibility for legally-residing immigrant children. |
| New Mexico | 2018 | Aged & Disabled (-): Home equity exclusion changed from the federal maximum of $840,000 to the federal minimum of $560,000 (Fewer than 5 individuals). |
| Ohio | 2017 | Aged & Disabled (#): Conversion from 209(b) to 1634 for SSI related groups. |
| Utah | 2017 | Children (+): Implementing the CHIPRA option to eliminate the 5-year bar on Medicaid eligibility for legally-residing immigrant children (Estimated to affect 750 children). |
| 2018 | Parents & Caretakers (+): Increased the Basic Maintenance Standard to 55% FPL (3,000 individuals). Adults (+) Pending Sec. 1115 Waiver: New eligibility group for chronically homeless, justice-involved individuals and those in need of substance abuse and/or mental health treatment, with income below 5% FPL. Adults (-) Pending Sec. 1115 Waiver: Add a work requirement for Primary Care Network (PCN) group. Adults (-) Pending Sec. 1115 Waiver: Eliminate of retroactive eligibility for PCN adults. Adults (-) Pending Sec. 1115 Waiver: Add 60-month limit on eligibility for PCN adults. Current Enrollees (-) Pending Sec. 1115 Waiver: Eliminate hospital presumptive eligibility. | |
| Virginia | 2017 | Disabled (+) Under Sec. 1115 Waiver: Increased eligibility from 60% to 80% FPL for waiver services for people with serious mental illness (GAP waiver program). (Note: had been decreased from 100% FPL to 60% FPL in FY 2016.) |
| 2018 | Disabled (+) Under Sec. 1115 Waiver: Increase eligibility from 80% to 100% FPL for waiver services for people with serious mental illness (GAP waiver program) (2,000 adults with SMI). (Full restoration to pre-2016 level.) | |
| Wyoming | 2018 | Adults (-): Income level for Breast and Cervical Cancer program reduced to 100% FPL (fewer than 50 individuals). Aged & Disabled (-): Income level for Employed Persons with Disabilities program reduced to 100% FPL (163 individuals). |
TABLE 3: CORRECTIONS-RELATED ENROLLMENT POLICIES IN ALL 50 STATES AND DC, FY 2017 AND FY 2018
| States | Medicaid Coverage For Inpatient Care Provided to Incarcerated Individuals | Medicaid Outreach/Assistance Strategies to Facilitate Enrollment Prior to Release* | Medicaid Eligibility Suspended Rather Than Terminated For Enrollees Who Become Incarcerated* | |||||||||
| Jails | Prisons | Jails | Prisons | Jails | Prisons | |||||||
| In place FY 2017 | New or Expanded FY 2018 | In place FY 2017 | New or Expanded FY 2018 | In place FY 2017 | New or Expanded FY 2018 | In place FY 2017 | New or Expanded FY 2018 | In place FY 2017 | New or Expanded FY 2018 | In place FY 2017 | New or Expanded FY 2018 | |
| Alabama | X* | X* | X* | X* | X* | X* | ||||||
| Alaska | X | X | X | X | X | X | ||||||
| Arizona | X | X | X | X | X | X | X | X | ||||
| Arkansas | X | X | X | X | X | X | ||||||
| California | X | X | X | X | X | X | ||||||
| Colorado | X | X | X | X | X | X | ||||||
| Connecticut | X | X | X | X | X | X | ||||||
| Delaware | X | X | X | X | X* | X* | ||||||
| DC | X | N/A | N/A | X | X | X | N/A | N/A | ||||
| Florida | X | X | ||||||||||
| Georgia | X | X | ||||||||||
| Hawaii | X | X | X | |||||||||
| Idaho | X | X | ||||||||||
| Illinois | X | X | X | X | ||||||||
| Indiana | X | X | X | X | X | X | ||||||
| Iowa | X | X | X | X | X | |||||||
| Kansas | X | X | ||||||||||
| Kentucky | X | X | X | X | X | X | ||||||
| Louisiana | X | X | X | X | X | X | X | |||||
| Maine | X | X | X | X | ||||||||
| Maryland | X | X | X | X | X | X | X | X | ||||
| Massachusetts | X | X | X | X | X | X | ||||||
| Michigan | X | X | X | X | X | X | ||||||
| Minnesota | X | X | X | X | X | X | ||||||
| Mississippi | X | X | X | |||||||||
| Missouri | X | X | X | X | ||||||||
| Montana | X | X | X | X | X | X | ||||||
| Nebraska | X | X | X | |||||||||
| Nevada | X | X | X | X | X | X | X | |||||
| New Hampshire | X | X | X | X | X | X | ||||||
| New Jersey | X | X | X | X | X | X | ||||||
| New Mexico | X | X | X | X | X | X | X | X | X | |||
| New York | X | X | X | X | X | X | ||||||
| North Carolina | X | X | ||||||||||
| North Dakota | X | X | X | |||||||||
| Ohio | X | X | X | X | X | |||||||
| Oklahoma | X | |||||||||||
| Oregon | X | X | X | X | X | X | ||||||
| Pennsylvania | X | X | X | X | X | X | X | X | X | X | ||
| Rhode Island | X | X | X | X | X | X | ||||||
| South Carolina | X | X | X | X | X | X | ||||||
| South Dakota | X | X | X | X | ||||||||
| Tennessee | X | X | X | X | ||||||||
| Texas | X | X | X | X | ||||||||
| Utah | X | X | X | X | ||||||||
| Vermont | X | X | X | X | ||||||||
| Virginia | X | X | X | X | X | |||||||
| Washington | X | X | X | X | X | X | X* | X* | ||||
| West Virginia | X | X | X | X | X | X | ||||||
| Wisconsin | X | X | X | X | ||||||||
| Wyoming | ||||||||||||
| Totals | 40 | 2 | 46 | 2 | 31 | 9 | 39 | 9 | 33 | 6 | 34 | 4 |
NOTES: ^States with “Medicaid outreach assistance strategies to facilitate enrollment prior to release” include those implementing a variety of strategies. In many cases, staff of the prison or jail provide most of the assistance in collaboration with the Medicaid agency. ^States that continue Medicaid eligibility for incarcerated individuals but limit covered benefits to inpatient hospitalization are also included in the count of states that suspend eligibility. “*” indicates that a policy was newly adopted in FY 2018, meaning that the state did not have any policy in that category/column in place in FY 2017. N/A: The District of Columbia has jails but no prisons. However, DC is counted under Medicaid outreach/assistance strategies because some individuals who serve prison terms outside of DC may be placed in residential re-entry centers upon returning to DC and may apply for Medicaid to access coverage for 24-hour inpatient care and to facilitate enrollment prior to release.SOURCE: Kaiser Family Foundation Survey of Medicaid Officials in 50 states and DC conducted by Health Management Associates, October 2017. | ||||||||||||
Report: Managed Care Initiatives
Key Section Findings
Managed care is the predominant delivery system for Medicaid in most states. Among the 39 states with comprehensive risk-based managed care organizations (MCOs), 29 states reported that 75 percent or more of their Medicaid beneficiaries were enrolled in MCOs as of July 1, 2017. Because of nearly full MCO saturation in most MCO states, only a few states reported actions to increase MCO enrollment. Although many states still carve-out behavioral health services from MCO contracts, movement to carve-in these services continues. Nearly all states have managed care quality initiatives in place such as pay for performance or capitation withholds.–What to watch:
- Twenty-six of the 39 MCO states reported that they plan to use authority to receive federal matching funds for adults receiving inpatient psychiatric or substance use disorder (SUD) treatment in an institution for mental disease (IMD) for no more than 15 days a month included in the 2016 managed care regulations. Close to half of MCO states reported that the day limit is insufficient to meet acute inpatient or residential treatment needs for those with serious mental illness (SMI) or SUD.34
- States are using MCO arrangements to increase attention to the social determinants of health and to promote value-based payment. States are increasingly requiring MCOs to screen beneficiaries for social needs (19 states in FY 2017 and 2 additional states in FY 2018); to provide care coordination pre-release to incarcerated individuals (6 states in FY 2017 and 1 additional state in FY 2018); and to use alternative payment models (APMs) to reimburse providers (13 states in FY 2017 set a target percentage of MCO provider payments that must be in an APM and 9 additional states plan to set targets in FY 2018).
Tables 4 through 8 include more detail on the populations covered under managed care (Tables 4 and 5), behavioral health services covered under MCOs (Table 6), managed care quality initiatives (Table 7), and minimum Medical Loss Ratio (MLR) policies (Table 8).
The Centers for Medicare and Medicaid Services (CMS) issued a final rule on managed care in Medicaid and CHIP in April 2016. The new rule represents a major revision and modernization of federal regulations in this area.35 36 On June 30, 2017, CMS released an Informational Bulletin37 indicating they would use “enforcement discretion” to work with states on achieving compliance with the new managed care regulations, except for specific areas that “have significant federal fiscal implications.”
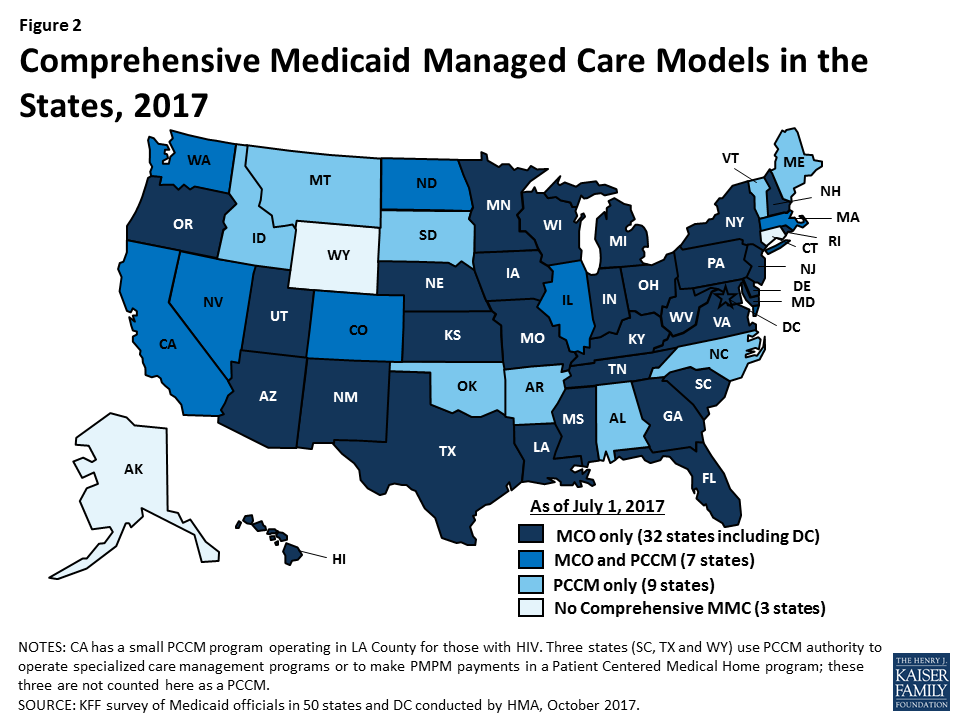
Managed care remains the predominant delivery system for Medicaid in most states. As of July 2017, all states except three – Alaska, Connecticut,38 and Wyoming– had some form of managed care in place, unchanged from July 2016. The number of states contracting with comprehensive risk-based managed care organizations (MCOs) (39 states) or operating a Primary Care Case Management (PCCM) program (16 states) as of July 2017 also remained unchanged from the prior year. PCCM is a managed fee-for-service (FFS) based system in which beneficiaries are enrolled with a primary care provider who is paid a small monthly fee to provide case management services in addition to primary care.
Of the 48 states that operate some form of managed care, seven operate both MCOs and a PCCM program while 32 states operate MCOs only and nine states operate PCCM programs only39 (Figure 2). Wyoming, one of the three states without any managed care (i.e., without either MCOs or a PCCM program), does operate a limited-benefit risk-based prepaid health plan (PHP). In total, 25 states (including Wyoming) contracted with one or more PHPs to provide Medicaid benefits including, behavioral health care, dental care, vision care, non-emergency medical transportation (NEMT), or long-term services and supports (LTSS).
Populations Covered by Risk-Based Managed Care
The share of Medicaid beneficiaries enrolled in MCOs or PCCM programs or remaining in FFS for their acute care varies widely by state. However, the share of Medicaid beneficiaries enrolled in MCOs has steadily increased as states have expanded their managed care programs to new regions and new populations and made MCO enrollment mandatory for additional eligibility groups. This year’s survey showed continued modest growth. The survey asked states to indicate the approximate share of specific Medicaid populations who receive their acute care in MCOs, PCCM programs, and FFS. As shown in Figure 3, among the 39 states with MCOs, 29 states reported that 75 percent or more of their Medicaid beneficiaries were enrolled in MCOs as of July 1, 2017 (up from 28 states in last year’s survey), including four of the five states with the largest total Medicaid enrollment. These four states (California, New York, Texas, and Florida) account for nearly four out of every 10 Medicaid beneficiaries across the country (Figure 3 and Table 4).40
Consistent with past survey results, this year’s survey found that children and adults (particularly those enrolled through the ACA Medicaid expansion) are much more likely to be enrolled in an MCO than elderly Medicaid beneficiaries or those with disabilities. Thirty-five of the 39 MCO states covered 75 percent or more of all children through MCOs. Twenty-eight of the 39 MCO states covered 75 percent or more of low-income adults in pre-ACA expansion groups (e.g., parents, pregnant women) through MCOs. The elderly and people with disabilities were the group least likely to be covered through managed care contracts, with only 16 of the 39 MCO states covering 75 percent or more such enrollees through MCOs (Figure 3).
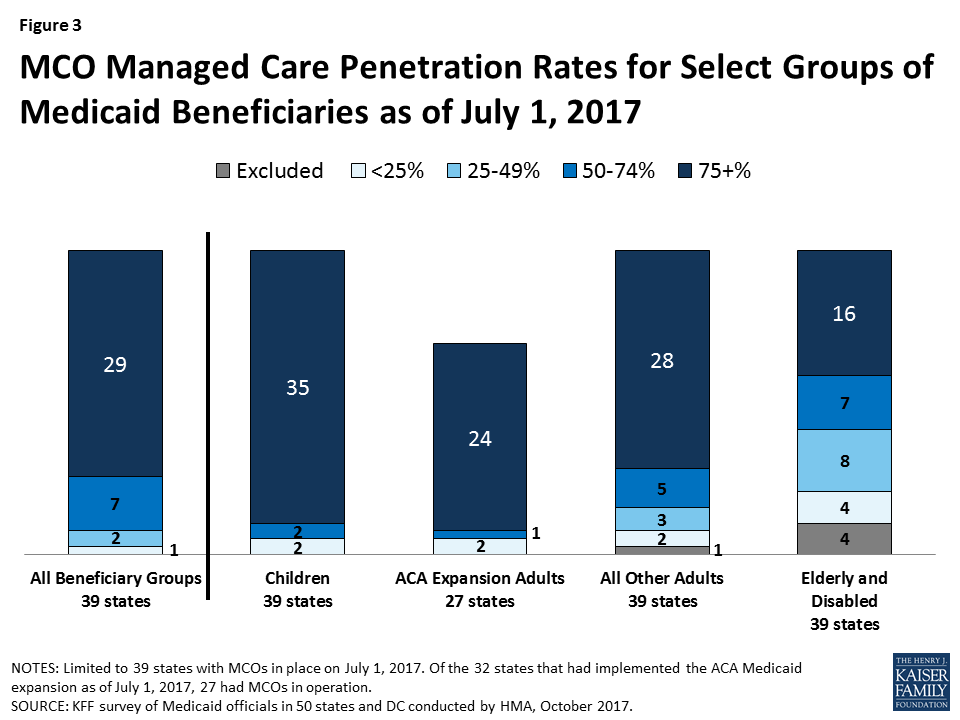
Of the 32 states that had implemented the ACA Medicaid expansion as of July 1, 2017, 27 were using MCOs to cover newly eligible adults. (The five Medicaid expansion states without risk-based managed care were Alaska, Arkansas, Connecticut, Montana, and Vermont.) The large majority (24) of these 27 states covered more than 75 percent of beneficiaries in this group through risk-based managed care. New Hampshire, however, reported that approximately 80 percent of its ACA expansion adults receive premium assistance to enroll in Qualified Health Plans in the state’s Marketplace and that only 13.5 percent were enrolled in MCOs. The other two states reporting less than 75 percent MCO penetration for this group were Colorado and Illinois.
Seven of the 16 states with PCCM programs also contract with MCOs. In most of these states, MCOs cover a larger share of beneficiaries than PCCM programs. However, Colorado and North Dakota are exceptions. As of July 1, 2017, a majority of Colorado’s enrollees were in the PCCM program, which is the foundation of the state’s Accountable Care Collaboratives, and 40 percent of enrollees in North Dakota were enrolled in the PCCM program (compared to 25 percent in the MCO program).
Only two states reported policies implemented in FY 2017 or planned for FY 2018 that reduced or will reduce the states’ reliance on the MCO model of managed care: Colorado reported that a small MCO pilot initiated on July 1, 2016 terminated on June 30, 2017, and Massachusetts reported that the implementation of its Accountable Care Organization (ACO) program in FY 2018 will result in the transition of MCO enrollees to ACOs.
Populations with Special Needs
For geographic areas where MCOs operate, this year’s survey asked MCO states whether, as of July 1, 2017, certain subpopulations with special needs were enrolled in MCOs for their acute care services on a mandatory or voluntary basis or were always excluded. On the survey, states selected from “always mandatory,” “always voluntary,” “varies,” or “always excluded” for the following populations: pregnant women, foster children, persons with intellectual and developmental disabilities (ID/DD), children with special health care needs (CSHCNs), persons with a serious mental illness (SMI) or serious emotional disorder (SED), and adults with physical disabilities. This year’s survey found an increase in the number of states reporting that enrollment for these populations is always mandatory (Exhibit 2).
As shown in Exhibit 2 and Table 5, and consistent with results in last year’s survey, pregnant women were the group most likely to be enrolled on a mandatory basis while persons with ID/DD were least likely to be enrolled on mandatory basis and also most likely to be excluded from MCO enrollment. Foster children were the group most likely to be enrolled on a voluntary basis, although they were enrolled on a mandatory basis in a larger number of states. Among states indicating that the enrollment approach for a given group or groups varied, LTSS eligibility was the most frequently cited basis of variation.
| Exhibit 2: MCO Enrollment of Populations with Special Needs, July 1, 2017 (# of States) | ||||||
| Pregnant women | Foster children | Persons with ID/DD | CSHCNs | Persons with SMI/SED | Adults w/ physical disabilities | |
| Always mandatory41 | 32 | 20 | 11 | 20 | 18 | 19 |
| Always voluntary | 2 | 8 | 4 | 3 | 3 | 4 |
| Varies | 4 | 8 | 16 | 14 | 16 | 11 |
| Always excluded | 1 | 3 | 8 | 2 | 2 | 5 |
Acute Care Managed Care Population Changes
In both FY 2017 and FY 2018, only a few states reported actions to increase enrollment in acute care managed care, reflecting full or nearly full MCO saturation in most MCO states. Of the 39 states with MCOs, a total of 14 states indicated that they made specific policy changes in either FY 2017 (7 states) or FY 2018 (8 states) to increase the number of enrollees in MCOs through geographic expansion, voluntary or mandatory enrollment of new groups into MCOs, or mandatory enrollment of specific eligibility groups that were formerly enrolled on a voluntary basis (Exhibit 3). Thirty-six states reported that acute care MCOs were operating statewide as of July 2017 and Illinois reported plans to expand MCOs statewide in FY 2018. The remaining two states without statewide MCO programs (Colorado and Nevada) did not report a geographic expansion planned for FY 2018.
| Exhibit 3: Medicaid Acute Care Managed Care Population Expansions, FY 2017 and FY 2018 | ||
| FY 2017 | FY 2018 | |
| Geographic Expansions | MO | IL |
| New Population Groups Added | LA, NE, OH, TX, WV | IL, NY, PA, TX |
| Voluntary to Mandatory Enrollment | WA | NY, OR, SC, VA, WI |
Some of the notable acute care MCO expansions include:
- West Virginia transitioned its SSI population from FFS to mandatory MCO enrollment in July 2016.
- Missouri extended its MCO program geographically statewide on May 1, 2017 for children, pregnant women, refugees, and custodial parents.
- In January 2018, Pennsylvania will begin to phase-in its Community HealthChoices program which will provide both physical health and long-term services and supports through newly contracted MCOs. CHC enrollees will include individuals in nursing facilities (currently carved out of managed care after 30 days), full benefit dually-eligible individuals, and individuals receiving home and community-based services.
In FY 2017 and FY 2018, states expanded MCO enrollment (either voluntary or mandatory) to additional groups. Some states added multiple groups. Some groups that states added or are planning to add include: foster care or adoption assistance children (New York, Ohio, and Texas); persons eligible for LTSS (Nebraska, New York, Ohio, Pennsylvania, and Texas); ACA expansion, newly eligible adult group and enrollees in the state’s Health Insurance Premium Payment Program (Louisiana); Breast and Cervical Cancer Treatment Program group (Ohio and Texas); children with special health care needs or SSI (Illinois, Ohio, and Texas); SSI population (West Virginia); and persons with intellectual and developmental disabilities (Nebraska and Ohio).
One state in FY 2017 and five in FY 2018 made enrollment mandatory for a specific eligibility group that was formerly enrolled on a voluntary basis: Washington (clients with Third Party Liability (TPL)/other insurance, excluding Medicare); New York (children with special health care needs in 1915(c) waiver programs); Oregon (Medicare/Medicaid dual eligibles); South Carolina (former foster care adults); Virginia (aged, blind, and disabled enrollees); and Wisconsin (SSI adults that do not have LTSS needs).
Services Covered Under MCO Contracts
Behavioral Health Services Covered Under MCO Contracts
Although MCOs are at risk financially for providing a comprehensive set of acute care services, nearly all states exclude or “carve-out” certain services from their MCO contracts, most commonly behavioral health services. In this year’s survey, states with acute care MCOs were asked to indicate whether specialty outpatient mental health (MH) services, inpatient mental health services, and outpatient and inpatient substance use disorder (SUD) services are always carved-in (i.e., virtually all services are covered by the MCO), always carved-out (to PHP or FFS), or carve-in status varies by geographic or other factors.
For purposes of this survey, “specialty outpatient mental health” services mean services used by adults with Serious Mental Illness (SMI) and/or youth with serious emotional disturbance (SED), commonly provided by specialty providers such as community mental health centers. Depending on the service, more than half of the 39 MCO states reported that specific behavioral health service types were carved into their MCO contracts, with specialty outpatient mental health services somewhat less likely to be carved in (Exhibit 4 and Table 6). Also, with the exception of inpatient SUD services, the number of states reporting that the other services were always carved-in increased modestly from last year.
| Exhibit 4: MCO Coverage of Behavioral Health, July 1, 2017 (# of States) | ||||
| Specialty Outpatient MH | Inpatient MH | Outpatient SUD | Inpatient SUD | |
| Always carved-in | 23 | 26 | 26 | 26 |
| Always carved-out | 11 | 8 | 8 | 7 |
| Varies | 5 | 5 | 5 | 6 |
States reporting actions in FY 2017 to carve in behavioral health services into their MCO contracts (in at least some regions/contracts) included six states (Minnesota, Nebraska, South Carolina, Texas, Virginia, and Washington). In FY 2018, ten states (Louisiana, Michigan, Minnesota, Mississippi, New Jersey, New York, Ohio, South Carolina, Washington, and West Virginia) report new/continued actions to carve in behavioral health services.
Institutions for Mental Diseases (IMD) Rule Change
The 2016 Medicaid Managed Care Final Rule42 allows states (under the authority for health plans to cover services “in lieu of” those available under the Medicaid state plan), to receive federal matching funds for capitation payments on behalf of adults who receive inpatient psychiatric or substance use disorder treatment or crisis residential services in an IMD for no more than 15 days in a month.43 States were asked in the survey whether they planned to use this new authority. Of the 39 states with MCOs, 26 states answered “yes” for FY 2017, FY 2018, or both FYs 2017 and 2018, five states answered “no,” and eight states answered “undetermined.”
States were also asked whether they believed the Final Rule allows MCOs sufficient flexibility to provide cost-effective “in lieu of” IMD services to meet acute inpatient or residential treatment needs for members with severe mental illness (SMI) or substance use disorders (SUDs). Only a small number of states (9 for SMI and 8 for SUD) answered “yes.” The remaining MCO states answered “no” (19 for SMI and 19 for SUD) or “don’t know” (11 for SMI and 12 for SUD). Many states commented that the 15-day limit was too restrictive, especially for SUD services, and a number of states indicated plans to seek a Section 1115 waiver to cover more than 15 days per month.44
Additional Services
States with MCO contracts reported that plans in their states may offer a range of services beyond those described in the state plan or waivers. Eleven states reported that MCOs provide limited or enhanced adult dental services beyond contractually required state plan benefits, and nine states reported enhanced vision services for adults. Several states noted that MCOs offer cell phones for reminder services or other technology supports from scales to telemedicine. Half of MCO states reported that MCOs provide a wide variety of non-clinical supports as value added services, including infant car seats and cribs, nominal gift cards for healthy behavior, air conditioners for asthma treatment, weight management classes or memberships, and even support for obtaining a GED. Health education, wellness supports, and non-traditional therapies were also noted by some states.
Managed Care (Acute and LTSS) Quality, Contract Requirements, and Administration
Quality Initiatives
Over time, the expansion of comprehensive risk-based managed care in Medicaid has been accompanied by greater attention to measuring quality and plan performance and, increasingly, to measuring health outcomes. After years of comprehensive risk-based managed care experience within the Medicaid program, many states now incorporate quality into the procurement process, as well as into ongoing program monitoring.
States procure MCO contracts using different approaches; however, most states use competitive bidding, in part because the dollar value is so large. Under these procurements, states can specify requirements and criteria that go beyond price, and may expect plans to compete on the basis of value-based payment arrangements with network providers, specific policy priorities such as improving birth outcomes, or strategies to address social determinants of health, and/or other specific performance and quality criteria. In this year’s survey, states were asked if they used, or planned to use, National Committee for Quality Assurance’s (NCQA’s) Healthcare Effectiveness Data and Information Set (HEDIS®) scores as criteria for selecting MCOs. Of the 39 states with MCOs, 18 indicated that they used or plan to use HEDIS scores as criteria for selecting MCOs.
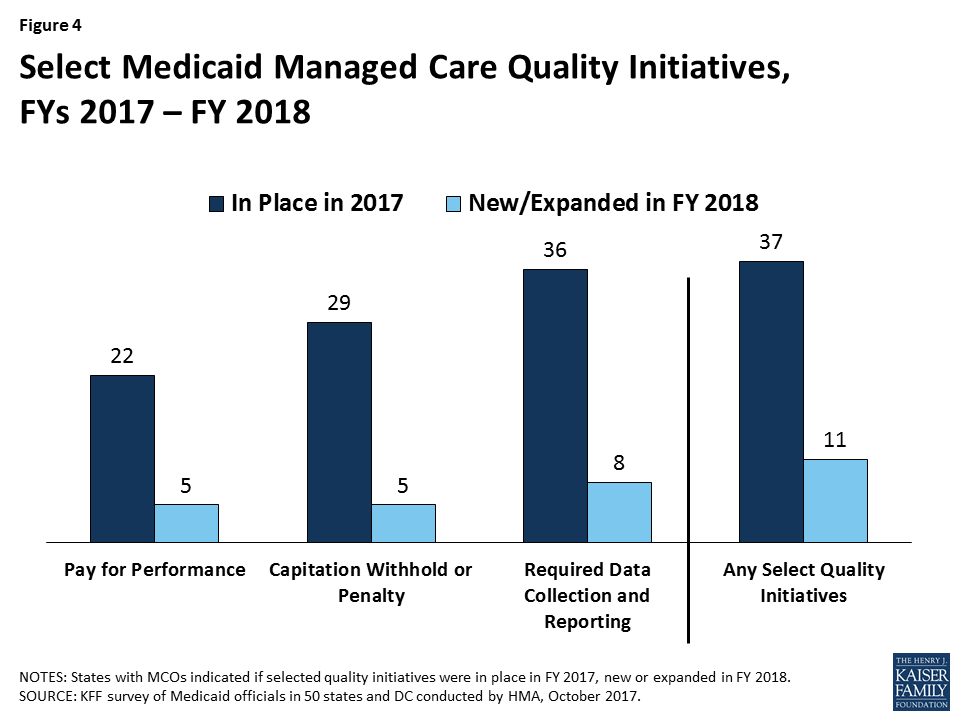
States were asked to indicate whether they had select quality strategies in place in FY 2017 and to indicate newly added or expanded initiatives for FY 2018. Thirty-seven MCO states reported one or more select MCO quality initiatives in place in FY 2017 (Figure 4 and Table 7). The most common strategies were requirements for data collection and reporting (often public reporting) on quality measures and the use of quality-based capitation withholds or penalties. Withhold amounts for acute care services typically ranged from 1 percent (Michigan, Oregon, and Washington) to 5 percent (Minnesota and Missouri); managed long-term services and supports (MLTSS) withhold amounts typically ranged from 0.5 percent (Iowa and Wisconsin) to 3 percent (California and Ohio). Over half the 39 states with managed care contracts reported the use of pay for performance strategies. In addition, several states reported “other” quality initiatives, including the use of liquidated damages for poor performance and the required operation of and reporting on performance improvement projects (PIPs).
In FY 2018, 11 states expect to implement new or expanded quality initiatives (Figure 4). The most common new quality initiatives are pay for performance initiatives while the most common expanded initiatives are data collection and reporting initiatives (Table 7).
Contract Requirements
Alternative [Provider] Payment Models within MCO Contracts
Value-based purchasing (VBP) strategies are important tools for states pursuing improved quality and outcomes and reduced costs of care within Medicaid and across payers. Generally speaking, VBP strategies include activities that hold a provider or MCO accountable for cost and quality of care.45 This often includes efforts to implement alternative payment models (APMs). APMs replace FFS/volume-driven provider payments with payment models that incentivize quality, coordination, and value (e.g., shared savings/shared risk arrangements and episode-based payments). Many states have included a focus on adopting and promoting APMs as part of their federally supported State Innovation Models (SIM) projects and as part of delivery system reform efforts approved under Section 1115 Medicaid waivers.46 States are increasingly encouraging or requiring Medicaid MCOs to adopt APMs to advance VBP in Medicaid. The survey found that:
- Thirteen states (Arizona, California, Delaware, Hawaii, Nebraska, New Hampshire, New Mexico, New York, Ohio, Pennsylvania, Rhode Island, South Carolina, and Washington) identified a specific target in their MCO contracts for the percentage of provider payments, network providers, or plan members that plans must cover via alternative provider payment models in FY 2017 (compared to five states in FY 2016); and
- Nine additional states (District of Columbia, Iowa, Kansas, Louisiana, Michigan, New Jersey, Oregon, Texas, and West Virginia) will include a target percentage in their contracts for FY 2018.
State APM Targets for Medicaid MCOs
- California’s Medi-Cal 2020 Waiver includes a requirement that MCOs must have VBP arrangements for 50-60 percent of all managed care lives assigned to receive care through a Designated Public Hospital participating in the PRIME program.47
- Delaware will require that 80 percent of all MCO members receive services from a provider under an alternative payment model by 2019.
- Iowa has a target of 40 percent for the share of an MCO’s membership to be covered by a VBP arrangement by FY 2018 and requires use of a common quality measurement tool.
- Washington requires MCOs to negotiate value-based contracts for at least 30 percent of capitated payments in FY 2017.
Further, in FY 2017, eight states had contracts that required Medicaid MCOs to adopt specific alternative provider payment models (e.g., episode of care payments, shared savings/shared risk, etc.), while eight states had contracts that encouraged MCOs to adopt specific APMs. In FY 2018, four additional states plan to require the use of specific APMs while five additional states plan to encourage use of specific APMs. The box below provides state examples of APM requirements.
State-Specific APM Requirements
- Minnesota MCOs are required to participate in the state’s ACO program, known as the Integrated Health Partnership. MCOs and the state share risk (gains and losses).
- Ohio requires MCOs to participate in Ohio’s retrospective episode-based payment model (with both positive and negative incentive payments) and the Ohio Comprehensive Primary Care program, Ohio’s patient-centered medical home (PCMH) program (with prospective, quarterly per-member per-month (PMPM) payments as well as retrospective total cost of care shared savings).
- Pennsylvania requires MCOs to make PCMH payments.
Social Determinants of Health
In April 2017, the CMS Center for Medicare and Medicaid Innovation selected 32 organizations to implement and test models to support local communities in addressing the health-related social needs of Medicare and Medicaid beneficiaries, aiming to bridge the gap between clinical and community service providers. This “Accountable Health Communities” model represents the first CMS innovation model that focuses on social determinants of health. The goal of the five-year program is to encourage innovation to deliver local solutions that improve access to community-based services.48 This development reflects growing awareness and interest on the part of CMS to address other issues, such as housing and food insecurity, by linking beneficiaries to social services and supports, ultimately to improve health and health outcomes. States have also focused on addressing social determinants of health, so federal and state activity are occurring simultaneously.
The survey found that, of the 39 MCO states, 19 states required while 12 states encouraged MCOs to screen enrollees for social needs and provide referrals to other services in FY 2017. Two additional states plan to require and two plan to encourage MCOs to screen/refer enrollees for social needs in FY 2018.
Strategies to Address Social Determinants of Health
- Arizona requires coordination of community resources like housing and utility assistance under its MLTSS contract. The state provides state-only funding in conjunction with its managed behavioral health contract to provide housing assistance. The state also encourages health plans to coordinate with the Veterans’ Administration and other programs to meet members’ social support needs.
- The District of Columbia encourages MCOs to refer beneficiaries with three or more chronic conditions to the “My Health GPS” Health Home program for care coordination and case management services, including a biopsychosocial needs assessment and referral to community and social support services.
- Louisiana requires screening for problem gaming and tobacco usage and requires referrals to Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) and the Louisiana Permanent Supportive Housing program when appropriate.
- Nebraska requires MCOs to have staff trained on social determinants of health and be familiar with community resources; plans are also required to have policies to address members with multiple biopsychosocial needs.
- New Jersey requires MLTSS plans to have a dedicated housing specialist and to provide assistance with attaining or maintaining housing as an “in lieu of” service.
Criminal Justice-Involved Populations
Engaging Medicaid MCOs in efforts to improve continuity of care for individuals released from correctional facilities into the community is important to ensure that individuals with complex or chronic health conditions, including behavioral health needs, have an effective transition to treatment in the community. In FY 2017, of the 39 states that contract with MCOs, six states (Arizona, Iowa, Kansas, Louisiana, Ohio, and Virginia) require MCOs to provide care coordination services to enrollees prior to release from incarceration, while two states (Kentucky and New Mexico) encourage MCOs to provide care coordination services prior to release. Five states intend to use contracts to encourage or require such care coordination in FY 2018. The following are examples of pre-release state requirements: Louisiana requires plans to offer care management at least 30 days prior to scheduled release; Kansas requires pre-release care management for LTSS populations, and new legislation in Washington will require care coordination pre-release and post-incarceration in FY 2018. New Mexico, a state that encourages but does not require pre-release activity, reported that one health plan is piloting a care coordination model through collaboration with a metropolitan detention center to test and learn effective methods to impact recidivism and improve public health and safety.
Administrative Policies
Minimum Medical Loss Ratios
The Medical Loss Ratio (MLR) is the proportion of total capitation payments received by an MCO spent on clinical services and quality improvement. CMS published a final rule in 2016 that requires states to develop capitation rates for Medicaid to achieve an MLR of at least 85 percent in the rate year, for rating periods and contracts starting on or after July 1, 2017.49 This 85 percent minimum MLR is the same standard that applies in Medicare Advantage and private large group plans. There is no federal requirement that Medicaid plans must pay remittances to the state if they fail to meet the MLR standard, but states have discretion to require remittances.
As of July 1, 2017, 25 of the 39 states that contract with comprehensive risk-based MCOs specified a minimum MLR in Medicaid MCO contracts. Twenty of these 25 states applied the MLR requirement to all MCO contracts, while five states applied it on a limited basis. Seventeen of the 25 states with minimum MLR requirements always require remittance payments to the state if the minimum MLR is not achieved; one state (Ohio) requires remittance under some circumstances.50
Medicaid MLRs vary by state but are most commonly set at 85 percent or higher. A few states noted that their minimum MLRs varied by type of plan or population. For example, in New Jersey, the MLR is calculated separately for each population covered, with an MLR of 85 percent set for acute care contracts and an MLR of 90 percent set for MLTSS contracts.
Table 8 provides state-specific information regarding the use of a minimum MLR.
Auto-Enrollment
Generally, beneficiaries who are required to enroll in MCOs must be offered a choice of at least two plans. Those who do not select a plan are auto-enrolled in a plan by the state. The proportion of MCO beneficiaries who are auto-enrolled varies widely across states. State auto-enrollment algorithms also vary, but usually take into consideration variables like previous plan or provider relationships, beneficiary geographic location, and/or plan enrollments of other family members. States may also aim to balance enrollment among plans. As of July 1, 2017, 11 states took plan quality or performance rankings into consideration in the auto-enrollment algorithm (Arizona, California, Hawaii, Michigan, Minnesota, New Mexico, New York, Ohio, South Carolina, Virginia, and Washington).
PCCM and PHP Program Changes
Primary Care Case Management (PCCM) Program Changes
Of the 16 states with PCCM programs, two reported enacting policies to increase PCCM enrollment in FY 2017 or FY 2018: Colorado reported continued growth in its PCCM-based Accountable Care Collaboratives in both FY 2017 and FY 2018, and Arkansas reported implementing new billing software that would auto-assign beneficiaries to a primary care physician. Two other states reported new PCCM programs: Alaska – one of only three states without either an MCO or PCCM program as of July 1, 2017 – reported plans to implement a PCCM program in FY 2018, and Arizona reported plans to implement an American Indian Medical Home effective October 1, 2017 using PCCM authority.
Three states51 (California, Illinois, and Massachusetts) reported actions to decrease enrollment in a PCCM program in FY 2017 or FY 2018. California plans to transition its one-county HIV PCCM program to a full-risk MCO model in CY 2018; Illinois reported that its Integrated Health Homes would move to managed care under new MCO contracts that would begin in FY 2018, and Massachusetts reported that implementation of its Accountable Care Organization (ACO) program in FY 2018 will include the transition of PCCM members to ACO Plans.
Limited-Benefit Prepaid Health Plans (PHP) Changes
In this year’s survey, the 25 states contracting with at least one PHP as of July 1, 2017, were asked to indicate whether certain services (listed in Exhibit 5 below) were provided under these arrangements. The most frequently cited services provided (of those included in the question) were outpatient mental health services (14 states), followed by non-emergency medical transportation (NEMT) (12 states) and inpatient mental health and outpatient substance use disorder (SUD) treatment services (11 states each).
| Exhibit 5: Services Covered Under PHP Contracts, July 1, 2017 | ||
| # of States | States52 | |
| Outpatient Mental Health | 14 | CA, CO, HI, ID, MA, MI, NC, OR, PA, TN, UT, WA, WI, WY |
| Inpatient Mental Health | 11 | CA, CO, HI, MA, MI, NC, PA, TN, UT, WA, WI |
| Outpatient Substance Use Disorder Treatment | 11 | CO, ID, MA, MI, NC, OR, PA, TN, UT, WA, WI |
| Inpatient Substance Use Disorder Treatment | 9 | CO, MA, MI, NC, PA, TN, UT, WA, WI |
| Non-Emergency Medical Transportation (NEMT) | 12 | FL, IA, KY, ME, MI, NJ, OK, RI, TN, TX, UT, WI |
| Dental | 9 | CA, IA, ID, LA, MI, RI, TN, TX, UT |
| Long-Term Services and Supports | 6 | ID, MI, NC, NY, TN, WI |
| Vision | 1 | TN |
Nine states reported implementing policies to increase PHP enrollment in FY 2017 or FY 2018. Five states (Arkansas, Iowa, Idaho, Nebraska, and Nevada) reported new or expanded dental PHPs in FY 2018, Indiana reported plans for an NEMT PHP contract in FY 2018, California reported adding inpatient SUD treatment to its PHP program in FY 2018, Louisiana will add “Coordinated System of Care” PHPs in FY 2018 (serving youth with behavioral health challenges and their families), and New York reported increased enrollment in its LTSS PHPs in both FY 2017 an FY 2018.
Four states also reported actions to decrease PHP enrollment in FY 2017 or FY 2018. Nebraska and Texas reported ending a behavioral health PHP and folding the covered services into MCO contracts in FY 2017. Washington reported that PHP enrollment decreased in FY 2017 and will decrease further in FY 2018 when the state converts behavioral health PHPs to fully integrated MCO contracts in additional geographic areas. Massachusetts reported that implementation of its Accountable Care Organization (ACO) program in FY 2018 will reduce enrollments in its behavioral health PHP program.
TABLE 4: SHARE OF THE MEDICAID POPULATION COVERED UNDER DIFFERENT DELIVERY SYSTEMS IN ALL 50 STATES AND DC, AS OF JULY 1, 2017
| States | Type(s) of Managed Care In Place | Share of Medicaid Population in Different Managed Care Systems | ||
| MCO | PCCM | FFS / Other | ||
| Alabama | PCCM | — | 86.4% | 13.6% |
| Alaska | FFS | — | — | 100.0% |
| Arizona | MCO | 93.1% | — | 6.9% |
| Arkansas* | PCCM | — | NR | NR |
| California | MCO and PCCM* | 78.9% | — | 21.1% |
| Colorado | MCO and PCCM* | 10.5% | 72.6% | 16.9% |
| Connecticut | FFS* | — | — | 100.0% |
| DC | MCO | 78.0% | — | 22.0% |
| Delaware | MCO | 94.2% | — | 5.8% |
| Florida | MCO | 92.0% | — | 8.0% |
| Georgia | MCO | 73.0% | — | 27.0% |
| Hawaii | MCO | 99.9% | — | <0.1% |
| Idaho* | PCCM | — | 95.0% | 5.0% |
| Illinois | MCO and PCCM | 63.4% | 10.4% | 26.2% |
| Indiana | MCO | 80.0% | — | 20.0% |
| Iowa | MCO | 92.6% | — | 7.4% |
| Kansas | MCO | 95.0% | — | 5.0% |
| Kentucky | MCO | 91.0% | — | 9.0% |
| Louisiana | MCO | 92.0% | — | 8.0% |
| Maine | PCCM | — | NR | NR |
| Maryland | MCO | 89.2% | — | 10.8% |
| Massachusetts | MCO and PCCM | 48.0% | 21.0% | 31.0% |
| Michigan | MCO | 74.5% | — | 25.5% |
| Minnesota | MCO | 76.0% | — | 24.0% |
| Mississippi | MCO | 70.0% | — | 30.0% |
| Missouri | MCO | 75.8% | — | 24.2% |
| Montana | PCCM | — | 72.0% | 28.0% |
| Nebraska | MCO | 99.6% | — | 0.4% |
| Nevada | MCO and PCCM | 72.0% | 6.0% | 22.0% |
| New Hampshire* | MCO | 73.0% | — | 4.1% |
| New Jersey | MCO | 95.8% | — | 4.2% |
| New Mexico | MCO | 88.7% | — | 11.3% |
| New York | MCO | 82.7% | — | 17.3% |
| North Carolina | PCCM | — | 90.0% | 10.0% |
| North Dakota | MCO and PCCM | 25.0% | 40.0% | 35.0% |
| Ohio | MCO | 88.5% | — | 11.5% |
| Oklahoma | PCCM | — | 75.1% | 24.9% |
| Oregon | MCO* | 89.0% | — | 11.0% |
| Pennsylvania | MCO | 82.3% | — | 17.7% |
| Rhode Island | MCO | 90.4% | — | 9.6% |
| South Carolina | MCO* | 76.0% | — | 24.0% |
| South Dakota | PCCM | — | 80.0% | 20.0% |
| Tennessee | MCO | 100.0% | — | 0.0% |
| Texas | MCO* | 91.7% | — | 8.1% |
| Utah | MCO | 84.9% | — | 15.1% |
| Vermont | PCCM | — | 63.0% | 37.0% |
| Virginia | MCO | 76.0% | — | 24.0% |
| Washington | MCO and PCCM | 85.0% | 2.0% | 13.0% |
| West Virginia | MCO | 80.0% | — | 20.0% |
| Wisconsin | MCO | 67.0% | — | 33.0% |
| Wyoming | FFS* | 0.2% | — | 99.8% |
| NOTES: NR – not reported. Share of Medicaid Population that is covered by different managed care systems. MCO refers to risk-based managed care; PCCM refers to Primary Care Case Management. FFS/Other refers to Medicaid beneficiaries who are not in MCOs or PCCM programs. *AR – Most Expansion Adults served by Qualified Health Plans through “Arkansas Works” premium assistance waiver. *CA – PCCM program operates in LA county for those with HIV. *CO – PCCM enrollees are part of the state’s Accountable Care Collaboratives (ACCs). *CT – terminated its MCO contracts in 2012 and now operates its program on a fee-for-service basis using four ASO entities. *ID – The Medicaid-Medicare Coordinated Plan (MMCP) has been recategorized by CMS as an MCO but is not counted here as such since it is secondary to Medicare. *NH – 22.9% of overall population and 80.1% of Expansion Adults are served by Qualified Health Plans under NH’s premium assistance program waiver *OR – MCO enrollees include those enrolled in the state’s Coordinated Care Organizations. *SC – uses PCCM authority to provide care management services to approximately 200 medically complex children. *TX – Texas Medicaid Wellness program provides care management services for high-cost/high-risk enrollees (under PCCM authority).*WY – the state does not operate a traditional PCCM or MCO program, but does use PCCM authority to make PCMH payments.SOURCE: Kaiser Family Foundation Survey of Medicaid Officials in 50 states and DC conducted by Health Management Associates, October 2017. | ||||
TABLE 5: ENROLLMENT OF SPECIAL POPULATIONS UNDER MEDICAID MANAGED CARE CONTRACTS FOR ACUTE CARE IN ALL 50 STATES AND DC, AS OF JULY 1, 2017
| States | Pregnant Women | Foster Children | Persons with ID/DD | CSHCNs | Persons with SMI/SED | Adults w/ physical disabilities |
| Alabama | — | — | — | — | — | — |
| Alaska | — | — | — | — | — | — |
| Arizona | Always mandatory | Always mandatory | Always mandatory | Always mandatory | Always mandatory | Always mandatory |
| Arkansas | — | — | — | — | — | — |
| California | Always mandatory | Varies | Varies | Always mandatory | Always mandatory | Always mandatory |
| Colorado | Always voluntary | Always voluntary | Always voluntary | Always voluntary | Always voluntary | Always voluntary |
| Connecticut | — | — | — | — | — | — |
| DC | Always mandatory | Varies | Always excluded | Varies | Varies | Varies |
| Delaware | Always mandatory | Always mandatory | Varies | Always mandatory | Always mandatory | Always mandatory |
| Florida | Always mandatory | Always mandatory | Always voluntary | Always mandatory | Always mandatory | Always mandatory |
| Georgia | Always mandatory | Always mandatory | Always excluded | Always excluded | Always excluded | Always excluded |
| Hawaii | Always mandatory | Always mandatory | Always mandatory | Always mandatory | Always mandatory | Always mandatory |
| Idaho | — | — | — | — | — | — |
| Illinois | Varies | Always excluded | Varies | Varies | Varies | Varies |
| Indiana | Always mandatory | Always voluntary | Varies | Varies | Varies | Varies |
| Iowa | Always mandatory | Always mandatory | Always mandatory | Always mandatory | Always mandatory | Always mandatory |
| Kansas | Always mandatory | Always mandatory | Always mandatory | Always mandatory | Always mandatory | Always mandatory |
| Kentucky | Varies | Varies | Varies | Varies | Varies | Varies |
| Louisiana | Always mandatory | Always mandatory | Varies | Always mandatory | Varies | Varies |
| Maine | — | — | — | — | — | — |
| Maryland | Always mandatory | Always mandatory | Varies | Varies | Varies | Always excluded |
| Massachusetts | Always voluntary | Always voluntary | Always voluntary | Always voluntary | Always voluntary | Always voluntary |
| Michigan | Always mandatory | Always mandatory | Always mandatory | Always mandatory | Always mandatory | Always mandatory |
| Minnesota | Always mandatory | Always voluntary | Always excluded | Always voluntary | Varies | Always voluntary |
| Mississippi | Always mandatory | Always voluntary | Varies | Varies | Varies | Varies |
| Missouri | Always mandatory | Always mandatory | Always excluded | Varies | Varies | Always excluded |
| Montana | — | — | — | — | — | — |
| Nebraska | Always mandatory | Always mandatory | Always mandatory | Always mandatory | Always mandatory | Always mandatory |
| Nevada | Always mandatory | Varies | Always excluded | Varies | Varies | Always excluded |
| New Hampshire | Always mandatory | Always mandatory | Always mandatory | Always mandatory | Always mandatory | Always mandatory |
| New Jersey | Varies | Always mandatory | Always mandatory | Always mandatory | Always mandatory | Always mandatory |
| New Mexico | Always mandatory | Always mandatory | Always mandatory | Always mandatory | Always mandatory | Always mandatory |
| New York | Always mandatory | Varies | Varies | Varies | Always mandatory | Always mandatory |
| North Carolina | — | — | — | — | — | — |
| North Dakota | Always excluded | Always excluded | Always excluded | Always excluded | Always excluded | Always excluded |
| Ohio | Always mandatory | Always mandatory | Varies | Varies | Varies | Always mandatory |
| Oklahoma | — | — | — | — | — | — |
| Oregon | Always mandatory | Varies | Varies | Always mandatory | Always mandatory | Always mandatory |
| Pennsylvania | Always mandatory | Always mandatory | Always mandatory | Always mandatory | Always mandatory | Always mandatory |
| Rhode Island | Always mandatory | Always mandatory | Varies | Always mandatory | Varies | Varies |
| South Carolina | Always mandatory | Always voluntary | Always excluded | Varies | Varies | Varies |
| South Dakota | — | — | — | — | — | — |
| Tennessee | Always mandatory | Always mandatory | Always mandatory | Always mandatory | Always mandatory | Always mandatory |
| Texas | Always mandatory | Always voluntary | Varies | Always mandatory | Always mandatory | Always mandatory |
| Utah | Varies | Varies | Varies | Varies | Varies | Varies |
| Vermont | — | — | — | — | — | — |
| Virginia | Always mandatory | Always mandatory | Varies | Varies | Varies | Varies |
| Washington | Always mandatory | Always voluntary | Varies | Always mandatory | Always mandatory | Always mandatory |
| West Virginia | Always mandatory | Always excluded | Always excluded | Always mandatory | Varies | Varies |
| Wisconsin | Always mandatory | Varies | Always voluntary | Varies | Always voluntary | Always voluntary |
| Wyoming | — | — | — | — | — | — |
| Always Mandatory | 32 | 20 | 11 | 20 | 18 | 19 |
| Always Voluntary | 2 | 8 | 4 | 3 | 3 | 4 |
| Varies | 4 | 8 | 16 | 14 | 16 | 11 |
| Always Excluded | 1 | 3 | 8 | 2 | 2 | 5 |
| NOTES: “–” indicates there were no MCOs operating in that state’s Medicaid program in July 2017. ID/DD – intellectual and developmental disabilities, CSHCN – Children with special health care needs, SMI – Serious Mental Illness, SED – Serious Emotional Disturbance. States were asked to indicate for each group if enrollment in MCOs is “always mandatory,” “always voluntary,” “varies,” or if the group is “always excluded” from MCOs as of July 1, 2017.SOURCE: Kaiser Family Foundation Survey of Medicaid Officials in 50 states and DC conducted by Health Management Associates, October 2017. | ||||||
TABLE 6: BEHAVIORAL HEALTH SERVICES COVERED UNDER ACUTE CARE MCO CONTRACTS IN ALL 50 STATES AND DC, AS OF JULY 1, 2017
| States | Specialty OP Mental Health | Inpatient Mental Health | Outpatient SUD | Inpatient SUD |
| Alabama | — | — | — | — |
| Alaska | — | — | — | — |
| Arizona | Varies | Varies | Varies | Varies |
| Arkansas | — | — | — | — |
| California | Always Carved-out | Always Carved-out | Always Carved-out | Always Carved-out |
| Colorado | Always Carved-out | Always Carved-out | Always Carved-out | Always Carved-out |
| Connecticut | — | — | — | — |
| DC | Always Carved-in | Always Carved-in | Always Carved-out | Always Carved-in |
| Delaware | Varies | Always Carved-in | Always Carved-in | Always Carved-in |
| Florida | Always Carved-in | Always Carved-in | Always Carved-in | Always Carved-in |
| Georgia | Always Carved-in | Always Carved-in | Always Carved-in | Always Carved-in |
| Hawaii | Always Carved-out | Always Carved-out | Always Carved-in | Always Carved-in |
| Idaho | — | — | — | — |
| Illinois | Always Carved-in | Always Carved-in | Always Carved-in | Always Carved-in |
| Indiana | Always Carved-out | Always Carved-in | Always Carved-in | Always Carved-in |
| Iowa | Always Carved-in | Always Carved-in | Always Carved-in | Always Carved-in |
| Kansas | Always Carved-in | Always Carved-in | Always Carved-in | Always Carved-in |
| Kentucky | Always Carved-in | Always Carved-in | Always Carved-in | Always Carved-in |
| Louisiana | Always Carved-in | Always Carved-in | Always Carved-in | Always Carved-in |
| Maine | — | — | — | — |
| Maryland | Always Carved-out | Always Carved-out | Always Carved-out | Always Carved-out |
| Massachusetts | Always Carved-in | Always Carved-in | Always Carved-in | Always Carved-in |
| Michigan | Always Carved-out | Always Carved-out | Always Carved-out | Always Carved-out |
| Minnesota | Always Carved-in | Always Carved-in | Always Carved-in | Always Carved-in |
| Mississippi | Always Carved-in | Always Carved-in | Varies | Varies |
| Missouri | Always Carved-out | Varies | Varies | Varies |
| Montana | — | — | — | — |
| Nebraska | Always Carved-in | Always Carved-in | Always Carved-in | Always Carved-in |
| Nevada | Always Carved-in | Always Carved-in | Always Carved-in | Always Carved-in |
| New Hampshire | Always Carved-in | Always Carved-in | Always Carved-in | Always Carved-in |
| New Jersey | Varies | Varies | Varies | Varies |
| New Mexico | Always Carved-in | Always Carved-in | Always Carved-in | Always Carved-in |
| New York | Always Carved-in | Always Carved-in | Always Carved-in | Always Carved-in |
| North Carolina | — | — | — | — |
| North Dakota | Always Carved-in | Always Carved-in | Always Carved-in | Always Carved-in |
| Ohio | Always Carved-out | Always Carved-out | Always Carved-out | Always Carved-out |
| Oklahoma | — | — | — | — |
| Oregon | Always Carved-in | Always Carved-in | Always Carved-in | Always Carved-in |
| Pennsylvania | Always Carved-out | Always Carved-out | Always Carved-out | Always Carved-out |
| Rhode Island | Always Carved-in | Always Carved-in | Always Carved-in | Always Carved-in |
| South Carolina | Always Carved-in | Varies | Always Carved-in | Always Carved-in |
| South Dakota | — | — | — | — |
| Tennessee | Always Carved-in | Always Carved-in | Always Carved-in | Always Carved-in |
| Texas | Always Carved-in | Always Carved-in | Always Carved-in | Always Carved-in |
| Utah | Always Carved-out | Always Carved-out | Always Carved-out | Always Carved-out |
| Vermont | — | — | — | — |
| Virginia | Always Carved-out | Always Carved-in | Always Carved-in | Always Carved-in |
| Washington | Varies | Varies | Varies | Varies |
| West Virginia | Always Carved-in | Always Carved-in | Always Carved-in | Varies |
| Wisconsin | Varies | Always Carved-in | Always Carved-in | Always Carved-in |
| Wyoming | — | — | — | — |
| Always Carved-in | 23 | 26 | 26 | 26 |
| Always Carved-out | 11 | 8 | 8 | 7 |
| Varies | 5 | 5 | 5 | 6 |
| NOTES: OP – Outpatient. SUD – Substance Use Disorder. “–” indicates there were no MCOs operating in that state’s Medicaid program in July 2017. For beneficiaries enrolled in an MCO for acute care benefits, states were asked to indicate whether these benefits are always carved-in (meaning virtually all services are covered by the MCO), always carved-out (to PHP or FFS), or whether the carve-in varies (by geography or other factor). “Specialty outpatient mental health” refers to services utilized by adults with Serious Mental Illness (SMI) and/or youth with serious emotional disturbance (SED) commonly provided by specialty providers such as community mental health centers.SOURCE: Kaiser Family Foundation Survey of Medicaid Officials in 50 states and DC conducted by Health Management Associates, October 2017. | ||||
TABLE 7: SELECT MEDICAID MANAGED CARE QUALITY INITIATIVES IN ALL 50 STATES AND DC, IN PLACE IN FY 2017 AND ACTIONS TAKEN IN FY 2018
| States | Pay for Performance/Performance Bonus | Capitation Withhold or Penalty | Required Data Collection and Reporting | Any Select Quality Initiatives | ||||||||
| In Place | New | Expanded | In Place | New | Expanded | In Place | New | Expanded | In Place | New | Expanded | |
| 2017 | 2018 | 2018 | 2017 | 2018 | 2018 | 2017 | 2018 | 2018 | 2017 | 2018 | 2018 | |
| Alabama | ||||||||||||
| Alaska | ||||||||||||
| Arizona | ||||||||||||
| Arkansas | ||||||||||||
| California | X | X | X | |||||||||
| Colorado | X | X | ||||||||||
| Connecticut | ||||||||||||
| DC | X | X | X | X | ||||||||
| Delaware | X | X | ||||||||||
| Florida | X | X | X | X | ||||||||
| Georgia | X | X | X | X | X | X | ||||||
| Hawaii | X | X | X | X | X | |||||||
| Idaho | ||||||||||||
| Illinois | X | X | X | X | ||||||||
| Indiana | X | X | X | X | ||||||||
| Iowa | X | X | X | X | ||||||||
| Kansas | X | X | X | X | ||||||||
| Kentucky | X | X | X | X | ||||||||
| Louisiana | X | X | X | X | X | X | ||||||
| Maine | ||||||||||||
| Maryland | X | X | X | X | ||||||||
| Massachusetts | X | X | X | |||||||||
| Michigan | X | X | X | X | ||||||||
| Minnesota | X | X | X | |||||||||
| Mississippi | X | X | ||||||||||
| Missouri | X | X | X | X | X | X | ||||||
| Montana | ||||||||||||
| Nebraska | X | X | X | X | X | X | ||||||
| Nevada | X | X | X | X | X | |||||||
| New Hampshire | X | X | X | X | X | X | ||||||
| New Jersey | X | X | X | X | ||||||||
| New Mexico | X | X | X | |||||||||
| New York | X | X | X | X | X | X | ||||||
| North Carolina | ||||||||||||
| North Dakota | ||||||||||||
| Ohio | X | X | X | X | ||||||||
| Oklahoma | ||||||||||||
| Oregon | X | X | X | X | ||||||||
| Pennsylvania | X | X | X | X | X | X | X | X | X | X | ||
| Rhode Island | X | X | X | X | ||||||||
| South Carolina | X | X | X | X | ||||||||
| South Dakota | ||||||||||||
| Tennessee | X | X | X | X | ||||||||
| Texas | X | X | X | X | X | |||||||
| Utah | X | X | ||||||||||
| Vermont | ||||||||||||
| Virginia | X | X | X | X | ||||||||
| Washington | X | X | X | |||||||||
| West Virginia | X | X | X | |||||||||
| Wisconsin | X | X | X | X | X | X | X | |||||
| Wyoming | ||||||||||||
| Totals | 22 | 4 | 2 | 29 | 2 | 4 | 36 | 2 | 7 | 37 | 5 | 8 |
| NOTES: States with MCO contracts were asked to report if select quality initiatives were included in contracts in FY 2017, or are new or expanded in FY 2018. The table does not reflect all quality initiatives states have included as part of MCO contracts.SOURCE: Kaiser Family Foundation Survey of Medicaid Officials in 50 states and DC conducted by Health Management Associates, October 2017. | ||||||||||||
TABLE 8: MINIMUM MEDICAL LOSS RATIO POLICIES FOR MEDICAID MCOs IN ALL 50 STATES AND DC, JULY 1, 2017
| Minimum Medical Loss Ratio (MLR) | ||||
| States | Require minimum MLR | % if required | Remittance required if MCO does not meet minimum MLR? | |
| Acute | LTSS | |||
| Alabama | — | |||
| Alaska | — | |||
| Arizona | Yes — always | 85% | 85% | No |
| Arkansas | — | |||
| California | No | |||
| Colorado | Yes — always | 85% | — | Yes — always |
| Connecticut | — | |||
| DC | Yes — always | 85% | — | No |
| Delaware | No | |||
| Florida | Yes — sometimes | 85% | N/A | No |
| Georgia | No | |||
| Hawaii | No | |||
| Idaho | — | |||
| Illinois | Yes — always | 85%* | 88% | Yes — always |
| Indiana | Yes — always | 85-87%* | — | Yes — always |
| Iowa | Yes — always | 88% | 88% | Yes — always |
| Kansas | No | |||
| Kentucky | Yes — always | 90% | — | Yes — always |
| Louisiana | Yes — always | 85% | — | Yes — always |
| Maine | — | |||
| Maryland | Yes — always | 85% | — | Yes — always |
| Massachusetts | Yes — sometimes | N/A | 80%* | No |
| Michigan | Yes — sometimes | 85% | N/A | No |
| Minnesota | No | |||
| Mississippi | Yes — always | 85% | — | Yes — always |
| Missouri | Yes — always | 85% | — | Yes — always |
| Montana | — | |||
| Nebraska | Yes — always | 85% | — | Yes — always |
| Nevada | Yes — always | 85% | — | Yes — always |
| New Hampshire | Yes — always | 89% | — | No |
| New Jersey | Yes — always | 85% | 90% | Yes — always |
| New Mexico | Yes — always | 86% | 86% | No |
| New York | No* | |||
| North Carolina | — | |||
| North Dakota | No | |||
| Ohio | Yes — sometimes | 85% | N/A | Yes — sometimes* |
| Oklahoma | — | |||
| Oregon | Yes — always | 80% | — | Yes — always |
| Pennsylvania | No | |||
| Rhode Island | No | |||
| South Carolina | Yes — sometimes | 86% | N/A | Yes — always |
| South Dakota | — | |||
| Tennessee | No | |||
| Texas | No* | |||
| Utah | No | |||
| Vermont | — | |||
| Virginia | Yes — always | 85% | 85% | Yes — always |
| Washington | Yes — always | 85-87%* | — | Yes — always |
| West Virginia | Yes — always | 85% | — | Yes — always |
| Wisconsin | No | |||
| Wyoming | — | |||
| Yes — always | 20 | 17 | ||
| Yes — sometimes | 5 | 1 | ||
| No | 14 | 7 | ||
| N/A – No MCOs | 12 | |||
| NOTES: In “Require Minimum MLR” column “–” indicates states that do not have Medicaid MCOs and “–” also appears in “LTSS %” column if state does not have MLTSS. “N/A” appears in “LTSS %” column if state with managed LTSS does not have LTSS MLR or in “Acute %” column if MCO state does not have acute MLR. *IL, IN, and WA indicated that the minimum acute MLR varies by population. *MA Senior Care Options (SCO) program has a minimum MLR of 80%. *NY is implementing MLR for acute and MLTSS in CY 2018 which will be effective retroactively to CY 2017. *TX has experience rebate on plans above a certain profit level.SOURCE: Kaiser Family Foundation Survey of Medicaid Officials in 50 states and DC conducted by Health Management Associates, 2017. | ||||
Report: Emerging Delivery System And Payment Reforms
Key Section Findings
In addition to managed care, Medicaid programs have been expanding their use of other service delivery and payment reform models to achieve better outcomes and lower costs. Forty states have one or more delivery system or payment reform initiatives in place in FY 2017 (e.g., patient-centered medical home (PCMH), ACA Health Home, accountable care organization (ACO), episode of care payment, or delivery system reform incentive program (DSRIP)).
What to watch: Six states reported episode of care initiatives in place in FY 2017 (up from only two states in FY 2015). For FY 2018, four of these states reported expanding these initiatives and three states reported new episode of care initiatives. Although states continue to show interest in DSRIP initiatives (that emerged under the Obama administration), the future of DSRIP remains unclear under the new administration. In response to two new survey questions, 19 states reported new or expanded initiatives to expand dental access or improve oral health outcomes (for children and/or adults) in FY 2017 or FY 2018 and 19 states also reported initiatives to expand the use of telehealth in FY 2018 or FY 2019.
Table 9 contains more detailed information on emerging delivery system and payment reform initiatives in place in FY 2017 and new or expanded initiatives in FY 2018.
This year’s survey asked states whether certain delivery system and payment reform models designed to improve health outcomes and constrain cost growth were in place in FY 2017, and whether they planned to adopt or enhance these models in FY 2018. Over three-quarters of all state Medicaid programs (40) had at least one of the specified delivery system or payment reform models in place in FY 2017 (Figure 5 and Table 9). If all actions reported by states for FY 2018 are implemented as planned, that number will grow to 44 states by the end of FY 2018, demonstrating the continued widespread and growing interest in Medicaid transformation. For FY 2018, a total of 22 states reported plans to adopt or expand one or more of the models to reward quality and encourage integrated care. Key initiatives include patient-centered medical homes (PCMHs), Health Homes, and Accountable Care Organizations (ACOs).
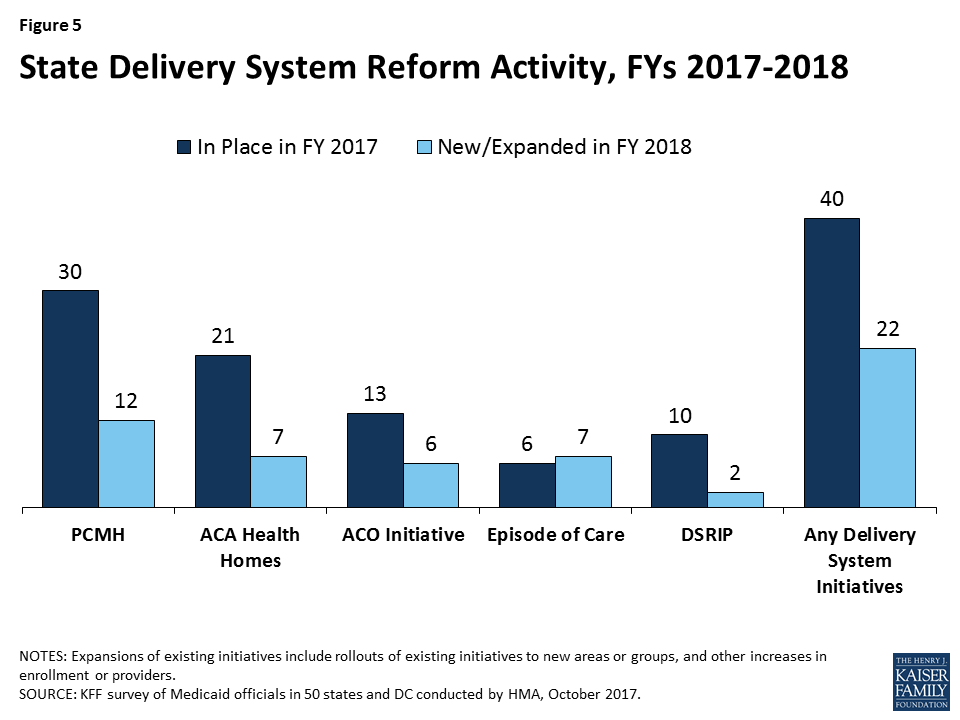
Patient-Centered Medical Homes (PCMHs)
PCMH initiatives operated in over half (30) of Medicaid programs in FY 2017 (Table 9). Under a PCMH model, a physician-led, multi-disciplinary care team holistically manages the patient’s ongoing care, including recommended preventive services, care for chronic conditions, and access to social services and supports. Generally, providers or provider organizations that operate as a PCMH seek recognition from organizations like the National Committee for Quality Assurance (NCQA).53 PCMHs are often paid (by state Medicaid agencies directly or through MCO contracts) a per member per month (PMPM) fee in addition to regular FFS payments for their Medicaid patients.54
In this year’s survey, 12 states reported plans to adopt or expand PCMHs in FY 2018 (Table 9). A few of these states reported notable expansions. Through its Centennial Care managed care program, New Mexico indicated that it now serves approximately 300,000 members through a PCMH and further expansion is planned for FY 2018. Tennessee reported launching its statewide multi-payer PCMH program (through its TennCare MCOs) with 29 organizations in January 2017 with plans to add practices each year. Wyoming, a state without MCO or PCCM programs, implemented PCMHs prior to FY 2017, but reported that it will continue to recruit and enroll physician practices into the program in FY 2018. Alaska, Delaware, and Illinois are planning to implement new PCMH initiatives in FY 2018 and Georgia’s MCO contracts will support expansion of the number of PCMHs in FY 2018.
In contrast, three states reported the restriction or elimination of a PCMH program: Massachusetts ended a PCMH program in December 2016 as part of its transition towards primary care-led reform through ACO models; Maine reported that the multi-payer PCMH program that it participated in was eliminated at the federal level, and South Carolina reported that it would be restricting its PCMH program by eliminating “level 1” funding after finding that many PCMHs remained in the application phase for more than 18 months.
ACA Health Homes
The ACA Health Homes option, created under Section 2703 of the ACA, builds on the PCMH concept. By design, Health Homes must target beneficiaries who have at least two chronic conditions (or one and risk of a second, or a serious and persistent mental health condition), and provide a person-centered system of care that facilitates access to and coordination of the full array of primary and acute physical health services, behavioral health care, and social and long-term services and supports. This includes services such as comprehensive care management, referrals to community and social support services, and the use of health information technology (HIT) to link services, among others. States receive a 90 percent federal match rate for qualified Health Home service expenditures for the first eight quarters under each Health Home State Plan Amendment; states can (and have) created more than one Health Home program to target different populations.
Over one-third of states (21) had at least one Health Home initiative in place in FY 2017 (Table 9). One of these states (Alabama) specifically noted that it is continuing to operate its Health Home initiative even though the eight quarters of enhanced federal funding has expired. In this survey, seven states also reported plans to adopt or expand Health Homes in FY 2018 (Table 9). Of these seven states, three (Alaska, California, and Illinois) reported new Health Home State Plan Amendments (SPAs) and four states (New Mexico, New York, Vermont, and West Virginia) reported expansions of existing Health Home programs. Ohio, however, reported plans to end its Health Home program in January 2018 as part of a behavioral health redesign initiative.
Accountable Care Organizations (ACOs)
Thirteen states reported having ACOs in place for at least some of their Medicaid beneficiaries in FY 2017 (Table 9).55 While there is no uniform, commonly accepted federal definition of an ACO, an ACO generally refers to a group of health care providers or, in some cases, a regional entity that contracts with providers and/or health plans, that agrees to share responsibility for the health care delivery and outcomes for a defined population.56 An ACO that meets quality performance standards that have been set by the payer and achieves savings relative to a benchmark can share in the savings. States use different terminology in referring to their Medicaid ACO initiatives, such as Regional Care Collaborative Organizations (RCCOs) in Colorado57 and Accountable Entities in Rhode Island.
In this survey, no state reported a new ACO initiative, but six states reported plans to expand an existing initiative in FY 2018 (Table 9). Four states with more mature ACO programs (Colorado, Maine, Minnesota, and Vermont) reported continued expansions of those programs in FY 2018, including Vermont, which also reported that it had aligned its existing ACO model with the design of the Medicare/CMS Next Generation ACO model beginning in January 2017. Two states (Massachusetts and Rhode Island) reported more significant expansions. Massachusetts reported that its ACO pilot program would be expanded statewide in January 2018 employing three different ACO models: an ACO/MCO partnership model; an ACO contracting directly with the state (without an MCO partner), and an exclusively MCO administered model. Massachusetts expects approximately 900,000 Medicaid beneficiaries to be enrolled in an ACO (out of 1.4 million total Medicaid enrollees). Rhode Island also reported plans to expand its current pilot “Accountable Entity” (AE) program in partnership with its existing MCOs with a long-term goal of having at least one-third of its Medicaid eligibles attributed to a certified AE.58
Episode-of-Care Payment Initiatives
Unlike FFS reimbursement, where providers are paid separately for each service, or capitation, where a health plan receives a PMPM payment for each enrollee intended to cover the costs for all covered services, episode-of-care payment provides a set dollar amount for the care a patient receives in connection with a defined condition or health event (e.g., heart attack or knee replacement). Episode-based payments usually involve payment for multiple services and providers, creating a financial incentive for physicians, hospitals, and other providers to work together to improve patient care and manage costs. Six states (Arkansas, New Mexico, New York, Ohio, Pennsylvania, and Tennessee) reported that they had episode-of-care payment initiatives in place in FY 2017, up from only two states in FY 2015 (Table 9). Four of these states (Arkansas, New Mexico, Ohio, and Tennessee) also reported planned expansions of these initiatives in FY 2018. Another three states (Alaska, Connecticut, and South Carolina) reported plans to implement a new episode-of-care initiative in FY 2018 (Table 9).
Delivery System Reform Incentive Payment (DSRIP) Program
DSRIP initiatives,59 which emerged under the Obama administration, provide states with significant federal funding to support hospitals and other providers in changing how they provide care to Medicaid beneficiaries.[endnote 239092-11] DSRIP initiatives link funding for eligible providers to process and performance metrics. Ten states reported DSRIP initiatives in place in FY 2017 (Table 9). Two of these states (Massachusetts and Texas) reported expansions planned for FY 2018: Massachusetts’ expanded DSRIP will support the development of ACOs and Texas reported new protocols for DSRIP activities, subject to CMS approval. Although states continue to show interest in pursuing delivery system reform through Section 1115 waiver authority, the future of DSRIP initiatives remains unclear under the new administration.
Other Initiatives
In addition to the initiatives discussed above, states mentioned a variety of other delivery system and payment reform initiatives (not counted in the totals for Figure 5 and Table 9). For example, DC reported on its pay for performance (P4P) initiatives including a new rate methodology for nursing facilities that includes P4P and an federally qualified health center (FQHC) P4P program focused on reducing inappropriate use of the emergency room, decreasing the rate of potentially avoidable hospital admissions, and addressing the problem of hospital readmissions. Montana reported on the implementation and expansion of a tribal health improvement program. Nevada reported implementing Certified Community Behavioral Health Clinics (CCBHCs) using an integrated behavioral health model, a prospective payment system, and pay for performance measures. Rhode Island reported using Section 1115 waiver authority to receive federal matching funds for services currently provided by various state agencies to support a new Designated State Health Program aimed at supporting quality-based payment programs, and Wisconsin reported plans to implement incentives to reduce potentially preventable hospital readmissions among both managed care and FFS members.
All-payer claims database (APCD) systems are large-scale databases that systematically collect medical claims, pharmacy claims, dental claims (typically, but not always), and eligibility and provider files from both private and public payers. APCDs can be used to help identify areas to focus reform efforts and for other purposes. Sixteen states (up from 11 in FY 2015) reported that an APCD was in place in their states (although Minnesota reported that the Medicaid program did not have access to the APCD in its state); one state (Connecticut) reported an APCD expansion planned for FY 2018; and three states (Delaware, Hawaii, and Washington) reported plans for new APCDs in FY 2018. Tennessee, however, reported that its APCD would be eliminated stating that following the United States Supreme Court’s 2016 decision in Gobeille v. Liberty Mutual Insurance Company,60 the Tennessee Attorney General opined that the statute authorizing the Tennessee APCD could no longer be enforced.
Access Improvement Focus Areas
This year’s survey included additional questions for states focusing on initiatives to increase access to dental care or improve oral health outcomes and initiatives to increase access to telehealth. States were asked to briefly describe initiatives implemented in FY 2017 or planned for FY 2018.
Improving Dental Access or Oral Health Outcomes
Oral health is a critical component of overall health and well-being, but the prevalence of dental disease and tooth loss is disproportionately high among people with low income, reflecting lack of access to dental coverage and care.61 While all state Medicaid programs are required to provide a comprehensive dental benefit for children, dental services remain an optional benefit for adults and securing an adequate number of dental providers is a challenge in many areas. In this year’s survey, 19 states described a variety of new or expanded initiatives to expand dental access or improve oral health outcomes (for children and/or adults) implemented in FY 2017 or FY 2018 (Exhibit 6).
| Exhibit 6: Strategies to Improve Dental Access or Oral Health Outcomes | ||
| # of States | States | |
| Payment incentives or value-based purchasing arrangements | 8 | CA, DC, MN, OH, OR, TX, WA, WI |
| Reimbursement rate increases (sometimes targeted) | 5 | CA, MN, OR, PA, WI |
| New or planned contracts with Dental Benefit Managers (DBMs) | 4 | AR, FL, NE, NV |
| Dental performance measures or Performance Improvement Projects (PIPs) within managed care | 4 | FL, MI, MO, OR |
| Consumer outreach/education campaigns | 2 | FL, MN |
In addition to the strategies noted in Exhibit 6 above, Pennsylvania reported expanded use of mid-level oral healthcare providers; Maryland reported expanded dental coverage to former foster care adults; and South Dakota reported working with its DBM vendor on care coordination initiatives (between primary care and dental care). Also see the “Benefit Changes” section of this report for details on states with dental benefit expansions. A number of other states mentioned ongoing initiatives implemented prior to FY 2017.
Improving Access to Care Through Telehealth
Interest in telehealth has grown across both public and commercial payers as a way to expand access to care, create greater convenience for patients, improve the quality of care, and reduce the costs of care. There are various types of telehealth services including: medical care/consultation between a patient at home and a distant clinician or between a patient in the presence of a clinician and a distant clinician; consultations between two clinicians without the patient present; remote monitoring of a patient at home or in a hospital or other facility; and secure electronic transfer of patient information (e.g., an image or lab results) to a clinician.62
In this year’s survey, 19 states reported initiatives to expand the use of telehealth in FY 2017 or FY 2018. Nine states reported expanding telehealth by covering additional services, diagnoses, or provider types (Arizona, Minnesota, Mississippi, Montana, and Washington), removing a 20 mile distance restriction (Indiana), adding or encouraging remote patient monitoring (Florida and Maryland) and distant site providers (Maryland), and allowing a patient’s home to be an acceptable patient site and clarifying that an initial in-person visit is not required if the telehealth service is being used to treat a behavioral health condition (Texas). California and Colorado reported telehealth pilot programs; Nevada indicated that its MCOs had implemented “NowClinics” to promote telehealth utilization, and South Dakota reported that it was working with tribal facilities to expand telehealth availability. Other states that reported new or expanded telehealth initiatives include Arkansas, Hawaii, Illinois, New Jersey, New Mexico, and New York.
TABLE 9: SELECT DELIVERY SYSTEM AND PAYMENT REFORM INITIATIVES IN ALL 50 STATES AND DC, IN PLACE IN FY 2017 AND ACTIONS TAKEN IN FY 2018
| States | Patient-Centered Medical Homes(PCMH) | ACA Health Homes | Accountable Care Organizations (ACO) | Episode of Care Payments | Delivery System Reform Incentive Payment Program(DSRIP) | Any Delivery System or Payment Reform Initiatives | ||||||
| In Place FY 2017 | New/ Expand FY 2018 | In Place FY 2017 | New/ Expand FY 2018 | In Place FY 2017 | New/ Expand FY 2018 | In Place FY 2017 | New/ Expand FY 2018 | In Place FY 2017 | New/ Expand FY 2018 | In place FY 2017 | New/Exp in FY 2018 | |
| Alabama | X | X | X | |||||||||
| Alaska | X* | X* | X* | X* | ||||||||
| Arizona | X | X | ||||||||||
| Arkansas | X | X | X | X | X | X | ||||||
| California | X* | X | X | X | ||||||||
| Colorado | X | X | X | X | X | X | ||||||
| Connecticut | X | X | X | X* | X | X | ||||||
| Delaware | X* | X* | ||||||||||
| DC | X | X | ||||||||||
| Florida | X | X | ||||||||||
| Georgia | X* | X* | ||||||||||
| Hawaii | ||||||||||||
| Idaho | X | X | ||||||||||
| Illinois | X* | X* | X* | |||||||||
| Indiana | ||||||||||||
| Iowa | X | X | X | X | ||||||||
| Kansas | X | X | ||||||||||
| Kentucky | ||||||||||||
| Louisiana | ||||||||||||
| Maine | X | X | X | X | ||||||||
| Maryland | X | X | ||||||||||
| Massachusetts | X | X | X | X | X | X | X | |||||
| Michigan | X | X | X | X | X | |||||||
| Minnesota | X | X | X | X | X | X | ||||||
| Mississippi | ||||||||||||
| Missouri | X | X | X | X | ||||||||
| Montana | X | X | ||||||||||
| Nebraska | X | X | ||||||||||
| Nevada | X | X | ||||||||||
| New Hampshire | X | X | ||||||||||
| New Jersey | X | X | X | X | ||||||||
| New Mexico | X | X | X | X | X | X | X | X | X | |||
| New York | X | X | X | X | X | X | X | X | ||||
| North Carolina | X | X | X | |||||||||
| North Dakota | ||||||||||||
| Ohio | X | X | X | X | X | X | X | |||||
| Oklahoma | X | X | X | |||||||||
| Oregon | X | X | ||||||||||
| Pennsylvania | X | X | X | X | X | X | X | |||||
| Rhode Island | X | X | X | X | X | X | ||||||
| South Carolina | X | X* | X | X | ||||||||
| South Dakota | X | X | ||||||||||
| Tennessee | X | X | X | X | X | X | X | |||||
| Texas | X | X | X | X | X | |||||||
| Utah | ||||||||||||
| Vermont | X | X | X | X | X | X | X | |||||
| Virginia | X | X | ||||||||||
| Washington | X | X | X | |||||||||
| West Virginia | X | X | X | X | ||||||||
| Wisconsin | X | X | X | |||||||||
| Wyoming | X | X | X | X | ||||||||
| Totals | 30 | 12 | 21 | 7 | 13 | 6 | 6 | 7 | 10 | 2 | 40 | 22 |
| NOTES: Expansions of existing initiatives include rollouts of existing initiatives to new areas or groups and significant increases in enrollment or providers. “*” indicates that a policy was newly adopted in FY 2018, meaning that the state did not have any policy in that category/column in place in FY 2017.SOURCE: Kaiser Family Foundation Survey of Medicaid Officials in 50 states and DC conducted by Health Management Associates, October 2017. | ||||||||||||
Report: Long-term Services And Supports Reforms
Key Section Findings
The vast majority of states in FY 2017 (47) and all states in FY 2018 are employing one or more strategies to expand the number of people served in home and community-based settings. The most common strategies include using HCBS waivers or state plan options, building rebalancing incentives into managed long-term services and supports (MLTSS) contracts, and serving more individuals through Programs of All-Inclusive Care for the Elderly (PACE) programs. Twenty-three states cover LTSS through one or more capitated managed care arrangements.
What to watch: Housing supports are an increasingly important part of state LTSS benefits. Over half of states (27) reported that they implemented or expanded housing-related activities outlined in CMS’s June 2015 Informational Bulletin63 (e.g., housing transition services, housing and tenancy sustaining services) in FY 2017 or FY 2018 (up from 16 states reported last year). In response to a new survey question about how states are addressing LTSS direct care workforce shortages and turnover, 17 states reported efforts in FY 2017 or FY 2018 to increase wages for direct care workers and/or engage in targeted workforce development activities (recruiting, training, credentialing, etc.).
Additional information on HCBS expansions implemented in FY 2017 or planned for FY 2018 as well as state-level details on capitated MLTSS models can be found in Tables 10 and 11.
Medicaid is the nation’s primary payer for long-term services and supports (LTSS), covering a continuum of services ranging from home and community-based services (HCBS) that allow persons to live independently in their own homes or in other community settings to institutional care provided in nursing facilities (NFs) and intermediate care facilities for individuals with intellectual disabilities (ICF-IDs). In 2015, spending on HCBS increased 7 percent while institutional spending decreased slightly, by 0.1 percent. HCBS represented 55 percent of total Medicaid expenditures on LTSS, and institutional LTSS represented 45 percent,64 a dramatic shift from 1995, when institutional settings accounted for 82 percent of national Medicaid LTSS expenditures.65
This year’s survey shows the vast majority of states in FY 2017 (47) and all states in FY 2018 (51) employing one or more strategies to expand the number of people served in home and community-based settings (Figure 6 and Table 10).
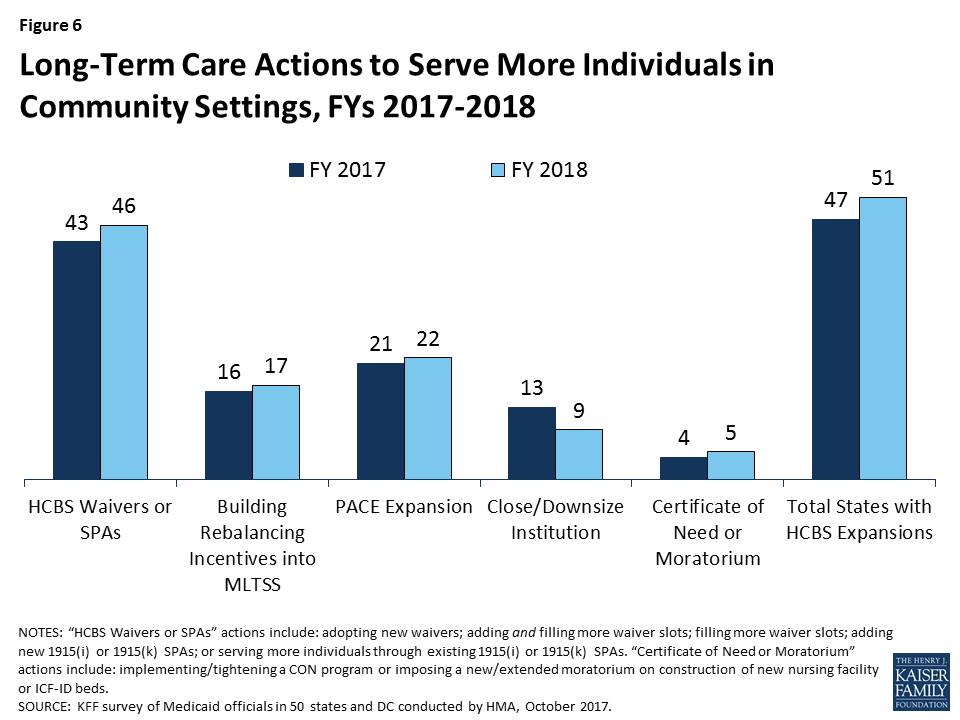
- Forty-three states in FY 2017 and 46 states in FY 2018 report using HCBS waivers and/or State Plan Amendments (SPAs) to expand the number of people receiving HCBS.66 HCBS waivers and SPAs include Section 1915(c) and Section 1115 waivers67 as well as Section 1915(i) and 1915(k) (“Community First Choice”) state plan options.
- Sixteen states in FY 201768 and 17 states in FY 2018 report including specific rebalancing incentives, performance targets and/or financial incentives, in managed care contracts to encourage MCOs that cover LTSS to expand access to HCBS.
- Twenty-one states in FY 2017 and 22 states in FY 2018 report serving more individuals through Programs of All-Inclusive Care for the Elderly (PACE) programs.69
- Thirteen states in FY 2017 and nine states in FY 2018 are closing or downsizing a state institution and transitioning residents into community settings.
- Four states in FY 2017 and five states in FY 2018 are implementing or tightening a Certificate of Need (CON) program or imposing or extending a moratorium on construction of new NF or ICF-ID beds.
States were also asked whether they have adopted or plan to adopt new restrictions on the number of people served in the community. In FY 2018, Missouri noted they are reducing enrollees’ state plan consumer directed services budgets to equal 60 percent of the cost of nursing home care. In addition, Texas noted that, while the state budget included increased funding to support additional slots in Section 1915(c) waivers, the state has slowed down the rollout of new slots because of unanticipated increases in Community First Choice expenditures.
Table 10 shows state use of LTSS rebalancing tools in FY 2017 and FY 2018.
Housing Supports
In June 2015, CMS issued an Informational Bulletin to clarify when and how Medicaid reimburses for certain housing-related activities, including individual housing transition services, individual housing and tenancy sustaining services, and state-level housing related collaborative activities.70 CMS’s intent was to assist states in designing benefits that support community integration for seniors, individuals with disabilities, and individuals experiencing chronic homelessness. Over half of states (27 states) reported that they implemented or expanded a housing-related strategy outlined in the CMS bulletin in FY 2017 and/or FY 2018 (Exhibit 7).
| Exhibit 7: States Implementing or Expanding Housing-Related Services Outlined in the CMS Informational Bulletin | ||
| FY 2017 only | FY 2018 only | Both FY 2017 and FY 2018 |
| DC, IN, KY, MA, MN, RI, TN, VT | CT, DE, GA, HI, IL, KS, UT | AZ, CA, FL, MD, MI, MS, NJ, NY, OH, PA, SC, WA |
Many of the services outlined in CMS’s Informational Bulletin were developed under the auspices of federal grant programs, including the Money Follows the Person (MFP) rebalancing demonstration. MFP is a federal grant program, enacted under the Deficit Reduction Act of 2005 and extended through September 2016 by the Affordable Care Act, which operated in 44 states.71 ,72 Enhanced federal funding under MFP has supported the transition of over 63,000 individuals from institutional to home and community-based long-term care settings as of December 2015.73 Under MFP, states identified the lack of affordable and accessible housing as a major barrier to assisting individuals to leave institutional settings of care.74 With MFP resources, many states have offered new housing related services, incorporated housing expertise within the Medicaid program to increase the likelihood of successful community living for persons who need supports, and engaged in strategic activities to assist in identifying and securing housing resources for individuals who choose HCBS.75
After September 2016, with CMS approval, states can continue to transition eligible individuals through 2018 and expend remaining MFP funds through federal FY 2020.76 As of July 2017, 30 states report that they currently offer housing-related services under a state plan, Section 1915(c) waiver, or Section 1115 waiver that the state intends to continue after the expiration of the MFP. However, 21 states report some services will likely be discontinued when MFP funding runs out. Examples of MFP services and activities that states may discontinue include: home delivered meals, vehicle modifications, independent living skills training, housing transition services, peer mentorship, and caregiver education, among others.
LTSS Direct Care Workforce
Many states are struggling to find sufficient numbers of trained direct care workers to meet the growing LTSS demand, including the demand for care in home and community-based settings.77 ,78 Low wages, few benefits, limited opportunities for career advancement, inadequate training, and high rates of worker injury are all factors that contribute to a workforce shortage and high workforce turnover among paid LTSS direct care workers. In this year’s survey, states were asked to describe any Medicaid initiatives intended to address LTSS direct care workforce shortages or turnover. Seventeen states report efforts underway in FY 2017 or FY 2018 related to wage increases for direct care workers or to workforce development (Exhibit 8).79
| Exhibit 8: Strategies to Address LTSS Direct Care Workforce Shortages & Turnover | ||
| # of States | States | |
| Wage Increases | 11 | IL, IN, KS, ME, MI, MT, NC, NH, NY, RI, UT |
| Workforce Development (including recruiting, training, credentialing etc.) | 6 | AZ, CT, MA, TN, WA, WI |
HCBS Benefit Changes
Fourteen states in FY 2017 and 13 states in FY 2018 reported a wide variety of HCBS benefit additions or expansions. HCBS benefits include those in Section 1915(c) waivers, under Section 1915(i) authority or Section 1915(k) authority (“Community First Choice” or “CFC”), and state plan personal care services, home health services, or private duty nursing, and PACE (Exhibit 9).80 Most HCBS benefit changes reported involve the addition of HCBS services to existing waiver or state plan programs. Examples of HCBS services added by states include assistive technology, home delivered and medically tailored meals, personal supports, unpaid caregiver training, housing transition services and tenancy supports, and supported employment.
Some states implemented new HCBS programs in FY 2017 or FY 2018. Six states report establishing eleven new PACE sites over the reporting period (Exhibit 9). In FY 2017, Tennessee added a new HCBS program for individuals with ID/DD under its Section 1115 waiver (Employment and Community First CHOICES). In FY 2018, Idaho will implement a new Section 1915(i) state plan HCBS option for children with a serious emotional disturbance, Pennsylvania will add a new Community Living waiver for individuals with ID/DD, and Wyoming will implement a new Section 1915(k) (CFC) state plan service.
Only one state, Oregon, reported eliminating an HCBS benefit, proposing to eliminate coverage for a live-in program in FY 2018.
| Exhibit 9: HCBS Benefit Enhancements or Additions | ||
| Benefit | FY 2017 | FY 2018 |
| HCBS Enhancements or Additions to Existing HCBS Authority | CA, KY, MA, MN, MS, NC, NE, PA, SC, SD, WI | CA, DE, NH, NY, PA, TX, UT, VA, WA |
| New PACE site | IN, LA | CA (2 sites), CO, IN, NC (2 sites), TX (3 sites) |
| New Section 1915(c), (i), or (k) | IN, TN | ID, PA, WY |
Washington’s LTSS Changes through Section 1115 Transformation Waiver
Washington is implementing LTSS eligibility and benefit package changes (described below) under its Section 1115 waiver to broaden the array of services available to individuals and to support unpaid family caregivers. The state hopes that these reforms will enable beneficiaries to stay at home and delay or avoid the need for more intensive care, while preserving quality of life, reducing costs, and avoiding the need for beneficiaries to impoverish themselves to access LTSS.
Medicaid Alternative Care (MAC) LTSS benefit package – MAC is a new LTSS benefit package option for Medicaid beneficiaries to support those living at home with assistance provided by unpaid family caregivers. MAC is only available to Medicaid beneficiaries eligible for but not receiving Medicaid-funded LTSS through the state plan or a Section 1915(c) waiver benefit package. MAC benefits include caregiver assistance services, caregiver training and education, specialized medical equipment and supplies, and health maintenance therapy supports.
Tailored Supports for Older Adults (TSOA) eligibility pathway for LTSS – TSOA will serve individuals who do not meet existing Medicaid financial eligibility criteria but who are “at-risk” of future Medicaid LTSS use. TSOA creates a new coverage group with access to a limited HCBS benefit package. MAC benefits include caregiver assistance services, caregiver training and education, specialized medical equipment and supplies, health maintenance therapy supports, and personal assistance.
Capitated Managed Long-Term Services and Supports (MLTSS)
As of July 1, 2017, almost half of states (23 states) covered LTSS through one or more of the following types of capitated managed care arrangements:
- Medicaid MCO covering Medicaid acute care and LTSS (18 states)
- PHP covering only Medicaid LTSS (6 states)
- MCO arrangement for dual eligible beneficiaries covering Medicaid and Medicare acute care and Medicaid LTSS services in a single contract under the federal Financial Alignment Demonstration (FAD) (10 states)
Of the 23 states that reported using one or more of these MLTSS models, nine states reported using two models, and one state (New York) reported using all three. Of the states with capitated MLTSS, 15 offered some form of MLTSS plan on a statewide basis for at least some LTSS populations as of July 1, 2017 (Table 11).
Ten states offered an MCO-based FAD (California, Illinois, Massachusetts, Michigan, New York, Ohio, Rhode Island, South Carolina, Texas, and Virginia) as of July 1, 2017.81 ,82 The FAD model involves a three-way contract between an MCO, Medicare, and the state Medicaid program.83 ,84 Massachusetts also operates an administrative alignment demonstration (without financial alignment) for dually eligible beneficiaries (Senior Care Options program). Minnesota only operates an administrative alignment demonstration (without financial alignment) for dually eligible beneficiaries (Minnesota Senior Health Options program).
Other states not participating in a formal demonstration have taken action to encourage improved coordination and integration of services for the dually eligible population under MCO arrangements. Eight states85 require Medicaid-contracting MCOs to be Medicare Dual Eligible Special Needs Plans (D-SNP)86 or Fully Integrated Dual Eligible (FIDE) Special Needs Plans,87 creating an opportunity for improved coordination and integration for beneficiaries. Five states88 encourage MCOs to be a D-SNP or a FIDE-SNP.
MLTSS Enrollment
For geographic areas where MLTSS operates, this year’s survey asked whether, as of July 1, 2017, certain populations were enrolled in MLTSS on a mandatory or voluntary basis or were always excluded. On the survey, states selected from “always mandatory,” “always voluntary,” “varies,” or “always excluded” for the following populations: seniors, persons with ID/DD, nonelderly adults with physical disabilities, and full benefit dual eligible individuals. As shown in Exhibit 10 below, seniors were most likely to be enrolled on a mandatory basis. Persons with ID/DD were excluded from enrollment in only three MLTSS states, though they were least likely to be enrolled on a mandatory basis. No state offering MLTSS always excludes full benefit dual eligible individuals from MLTSS enrollment.
| Exhibit 10: MLTSS Enrollment by Populations, July 1, 2017 (# of States) | ||||
| Seniors | Persons with ID/DD | Nonelderly Adults with Physical Disabilities | Full Benefit Dual Eligibles | |
| Always mandatory | 13 | 7 | 11 | 11 |
| Always voluntary | 5 | 5 | 4 | 6 |
| Varies | 4 | 8 | 6 | 6 |
| Always excluded | 1 | 3 | 2 | 0 |
MLTSS Population Changes
Growth in the use of MLTSS has continued since the prior survey reporting year. South Carolina expanded MLTSS to new regions in FY 2017 while Pennsylvania will expand to new regions in FY 2018 (Exhibit 11). In FY 2018, Virginia will roll out a new MLTSS program. Wisconsin’s Family Care Program will be statewide by the end of FY 2018. Five states extended MLTSS to new populations in FY 2017 (Illinois, New York, South Carolina, Tennessee, and Texas). In FY 2018, two states (New York and Pennsylvania) will extend MLTSS to new populations. No states reported actions or plans to decrease the number of enrollees served in MLTSS in FY 2017 or FY 2018.
| Exhibit 11: MLTSS Population Expansions, FY 2017 and FY 2018 | ||
| FY 2017 | FY 2018 | |
| Geographic Expansions | SC, WI | PA, VA, WI |
| New Population Groups Added | IL, NY, SC, TN, TX | NY, PA |
MLTSS Benefits
Almost every MLTSS state (21 out of 23 states) includes both institutional and HCBS in the same contractual arrangement, while two states (Michigan and Tennessee) report that this varies by MLTSS arrangement. Only one state reported a MLTSS benefit change in FY 2017 or FY 2018. Michigan added hospice benefits to its FAD in FY 2017.
TABLE 10: LONG-TERM CARE ACTIONS TO SERVE MORE INDIVIDUALS IN COMMUNITY SETTINGS IN ALL 50 STATES AND DC, FY 2017 AND FY 2018
| States | Sec. 1915(c) or Sec. 1115 Waiver | Sec. 1915(i) HCBS State Plan Option | Sec. 1915(k) “Community First Choice” Option | Building Rebalancing Incentives into MLTSS | PACE Expansion | Close/ Downsize Institution | Certificate of Need or Moratorium | Total States with HCBS Expansions | ||||||||
| 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | |
| Alabama | X | X | X | X | ||||||||||||
| Alaska | X | X | ||||||||||||||
| Arizona | X | X | X | X | ||||||||||||
| Arkansas | X | X | X | X | X | X | ||||||||||
| California | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| Colorado | X | X | X | X | X | X | X | X | ||||||||
| Connecticut | X | X | X | X | X | X | X | X | X | X | ||||||
| DC* | X | X | ||||||||||||||
| Delaware | X | X | X | X | X | X | X | X | X | X | ||||||
| Florida | X | X | X | X | X | X | X | X | ||||||||
| Georgia | X | X | X | X | ||||||||||||
| Hawaii | X | X | X | X | ||||||||||||
| Idaho | X | X | X | X | X | X | X | |||||||||
| Illinois | X | X | X | X | X | X | ||||||||||
| Indiana | X | X | X | X | X | X | X | X | X | X | ||||||
| Iowa | X | X | X | X | X | X | X | X | ||||||||
| Kansas | X | X | X | X | ||||||||||||
| Kentucky | X | X | X | X | ||||||||||||
| Louisiana | X | X | X | X | ||||||||||||
| Maine | X | X | X | X | ||||||||||||
| Maryland | X | X | X | X | X | |||||||||||
| Massachusetts | X | X | X | X | X | X | X | |||||||||
| Michigan | X | X | X | X | X | X | X | X | X | |||||||
| Minnesota | X | X | X | X | X | X | ||||||||||
| Mississippi | X | X | X | X | X | |||||||||||
| Missouri | X | X | X | X | ||||||||||||
| Montana | X | X | X | X | X | X | X | |||||||||
| Nebraska | X | X | X | X | X | X | ||||||||||
| Nevada | X | X | X | |||||||||||||
| New Hampshire | X | X | X | X | X | |||||||||||
| New Jersey | X | X | X | X | X | X | X | X | ||||||||
| New Mexico | X | X | X | X | X | X | ||||||||||
| New York | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| North Carolina | X | X | X | X | X | X | ||||||||||
| North Dakota | X | X | X | X | X | X | X | X | X | X | ||||||
| Ohio | X | X | X | X | X | X | X | X | ||||||||
| Oklahoma | X | X | X | X | X | X | ||||||||||
| Oregon | X | X | X | X | X | X | X | X | ||||||||
| Pennsylvania | X | X | X | X | X | X | X | X | X | X | ||||||
| Rhode Island | X | X | X | X | ||||||||||||
| South Carolina | X | X | X | X | X | |||||||||||
| South Dakota | X | X | X | X | ||||||||||||
| Tennessee | X | X | X | X | X | X | X | X | X | |||||||
| Texas | X | X | X | X | X | X | X | X | X | X | ||||||
| Utah | X | X | X | X | ||||||||||||
| Vermont | X | X | X | X | ||||||||||||
| Virginia | X | X | X | X | X | X | X | X | X | |||||||
| Washington | X | X | X | X | X | X | X | X | ||||||||
| West Virginia | X | X | ||||||||||||||
| Wisconsin | X | X | X | X | X | X | X | |||||||||
| Wyoming | X | X | X | X | X | X | X | |||||||||
| Totals | 41 | 42 | 8 | 13 | 8 | 10 | 16 | 17 | 21 | 22 | 13 | 9 | 4 | 5 | 47 | 51 |
| NOTES: “1915(c) or Sec. 1115 Waiver” actions include: adopting new waivers; adding and filling more waiver slots; or filling more waiver slots. Actions under “1915(i) and 1915(k)” include adding new 1915(i) or 1915(k) SPAs or serving more individuals through existing 1915(i) or 1915(k) SPAs. “Certificate of Need or Moratorium” actions include: implementing/tightening a CON program or imposing a new/extended moratorium on construction of new nursing facility or ICF-ID beds. *DC – Although not reflected in the table/counts above, DC also reported implementing a uniform assessment tool and increasing the availability of Medicaid application assistance, streamlining the eligibility and enrollment process. Several states also highlighted continued rebalancing efforts through the Money Follows the Person (MFP) program; although this federal grant program ended in September 2016, with CMS approval, states can continue to transition eligible individuals through 2018 and expend remaining MFP funds through federal FY 2020.SOURCE: Kaiser Family Foundation Survey of Medicaid Officials in 50 states and DC conducted by Health Management Associates, October 2017. | ||||||||||||||||
TABLE 11: CAPITATED MLTSS MODELS IN ALL 50 STATES AND DC, AS OF JULY 1, 2017
| States | Medicaid MCO | PHP | Medicare + Medicaid Demonstration | Any MLTSS | Statewide |
| Alabama | |||||
| Alaska | |||||
| Arizona | X | X | X | ||
| Arkansas | |||||
| California | X | X | X | ||
| Colorado | |||||
| Connecticut | |||||
| DC | |||||
| Delaware | X | X | X | ||
| Florida | X | X | X | ||
| Georgia | |||||
| Hawaii | X | X | X | ||
| Idaho | X | X | |||
| Illinois | X | X | X | ||
| Indiana | |||||
| Iowa | X | X | X | ||
| Kansas | X | X | X | ||
| Kentucky | |||||
| Louisiana | |||||
| Maine | |||||
| Maryland | |||||
| Massachusetts | X | X* | X | ||
| Michigan | X | X | X | X | |
| Minnesota* | X | X | X | ||
| Mississippi | |||||
| Missouri | |||||
| Montana | |||||
| Nebraska | |||||
| Nevada | |||||
| New Hampshire | |||||
| New Jersey | X | X | X | ||
| New Mexico | X | X | X | ||
| New York | X | X | X | X | X |
| North Carolina | X | X | X | ||
| North Dakota | |||||
| Ohio | X | X | X | ||
| Oklahoma | |||||
| Oregon | |||||
| Pennsylvania | |||||
| Rhode Island | X | X | X | X | |
| South Carolina | X | X | |||
| South Dakota | |||||
| Tennessee | X | X | X | X | |
| Texas | X | X | X | X | |
| Utah | |||||
| Vermont | |||||
| Virginia | X | X | |||
| Washington | |||||
| West Virginia | |||||
| Wisconsin | X | X | X | ||
| Wyoming | |||||
| Totals | 18 | 6 | 10 | 23 | 15 |
| NOTES: States were asked whether they cover long-term services supports through any of the following managed care (capitated) arrangements as of July 1, 2017: Medicaid MCO (MCO covers Medicaid acute + Medicaid LTSS); PHP (covers only Medicaid LTSS); or Medicare + Medicaid Demonstration (Medicaid MCO covers Medicaid and Medicare acute + Medicaid LTSS). “Medicare + Medicaid Demonstration” – these states use Medicaid MCOs in Financial Alignment Demonstration (FAD) initiatives which involve care coordination for dually eligible beneficiaries. States were also asked whether MLTSS plans were operating in all regions of the state as of July 1, 2017 (statewide). *MA operates a FAD and another administrative alignment demonstration for dually eligible beneficiaries. *MN operates an administrative alignment demonstration (without financial alignment) for dually eligible beneficiaries. *OH offers a Medicaid MCO (MCO offers Medicaid acute + Medicaid LTSS) only in those counties where the FAD is offered; dually eligible seniors who opt out of the FAD must enroll in this Medicaid MCO model for Medicaid services.SOURCE: Kaiser Family Foundation Survey of Medicaid Officials in 50 states and DC conducted by Health Management Associates, 2017. | |||||
Report: Provider Rates And Taxes
Key Section Findings
Provider rate changes are often tied to the economy. In FY 2017 and FY 2018, with relatively stable economic conditions in most states, more states made, or are planning, provider rate increases compared to restrictions. This holds true across provider types, except for inpatient hospital rates (hospital rate restrictions are primarily rate freezes, which are counted as restrictions in this report). All states except Alaska rely on provider taxes and fees to fund a portion of the non-federal share of the costs of Medicaid. Three states indicate plans for new provider taxes in FY 2018 and 13 states plan at least one provider tax increase.
What to watch:
- About half of MCO states (18 of 39) require MCO rates to follow FFS rate changes for some provider types and two states (Louisiana and Mississippi) require MCO rates to be tied to FFS for all providers. Twenty-four states reported they had MCO rate floors for some provider types, and five states reported they had minimum MCO payment requirements for all types of Medicaid providers.
- Federal legislation recently under consideration in the Senate proposed to phase down the limit on state use of provider taxes (the “safe harbor threshold”) from the current allowable level, 6.0 percent of net patient revenues, to 5.0 percent of net patient revenues by FY 2025 in one proposal and 4.0 percent by FY 2025 in another. In this year’s budget survey, 29 states reported having at least one provider tax exceeding 5.5 percent of net patient revenues and 46 states reported having at least one provider tax exceeding 3.5 percent as of July 1, 2017. The data suggests that these federal proposals would restrict states’ ability to supply the non-federal share to finance Medicaid and could therefore shift additional costs to states.
Tables 12 through 14 provide complete listings of Medicaid provider rate changes and provider taxes and fees in place in FY 2017 and FY 2018.
Provider Rates
Provider rate changes are often tied to the economy. During economic downturns and budget shortfalls, states often turn to rate restrictions to contain costs and are more likely to increase rates during periods of recovery and revenue growth. This report examines rate changes across major provider categories: inpatient hospital, nursing facilities, MCOs, outpatient hospital, primary care physicians, specialists, dentists, and home and community-based services (HCBS). States were asked to report aggregate rate changes for each provider category in their FFS programs. In FY 2017, more states implemented rate increases for at least one category of providers (46 states) compared to rate restrictions (37 states) (Figure 7 and Table 12). Compared with what states projected for FY 2017 on last year’s survey, this year’s survey responses showed that six more states implemented rate increases in FY 2017 and four fewer states implemented rate restrictions.
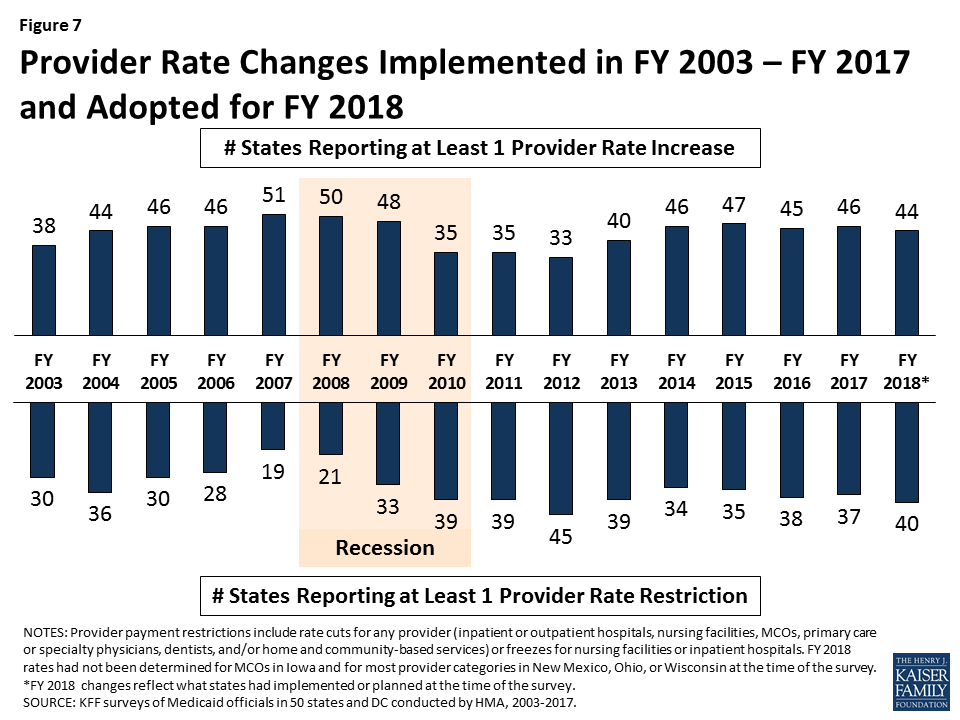
For FY 2018, the number of states with at least one implemented or planned rate increase (44 states) is greater than the number of states with at least one implemented or planned rate restriction (40 states) (Table 13).
The number of states with rate increases exceeded the number of states with restrictions in FY 2017 and FY 2018 across all major categories of providers (physicians, MCOs, and nursing facilities) with the exception of rates for inpatient hospital services89 (Figure 8). For the purposes of this report, cuts or freezes in rates for inpatient hospitals and nursing facilities are counted as restrictions.90 Most of the restrictions are for rate freezes. Four states in FY 2017 and five states in FY 2018 had implemented or planned reductions to inpatient hospital rates; only one state cut nursing facility rates in FY 2017, and two states plan to cut nursing facility rates in FY 2018.
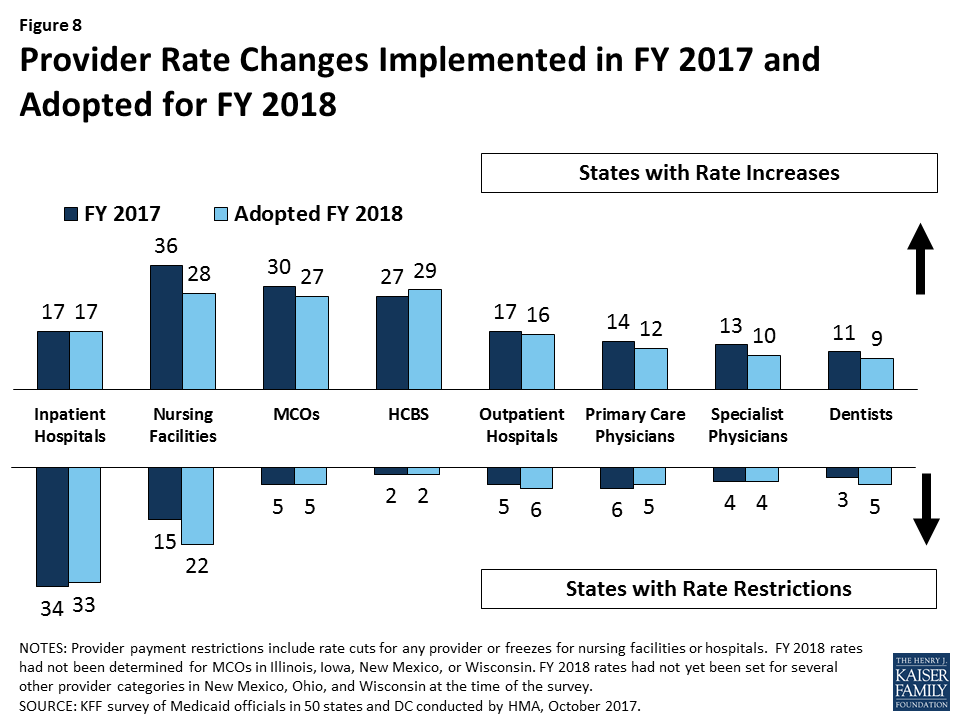
The number of states planning to increase nursing facility rates dropped in FY 2018 (28) compared to FY 2017 (36 states). HCBS providers were among those most likely to receive rate increases (27 states in FY 2017 and 29 states in FY 2018).
Capitation payments for MCOs are generally bolstered by the federal requirement that states pay actuarially sound rates. In FY 2017 and FY 2018, the majority of the 39 states with Medicaid MCOs either implemented or planned increases in MCO rates. Five states reported MCO rate cuts in FY 2017, and five states plan to cut MCO rates in FY 2018. Four states were not able to report MCO rate changes for FY 2018 because rate development was not complete. States are increasingly moving to calendar year MCO contracts.
Tables 12 and 13 provide state level details on provider rate changes in FY 2017 and FY 2018.
MCO Rate Requirements
In many states, MCOs make most of the Medicaid payments to providers. States were asked whether they require their MCOs to make changes to their provider payments when the state makes changes to FFS rates (such as rate increases). Of the 39 states with MCOs, 19 states indicated that they had no such requirement, 18 states have such a requirement for some provider types, and two states (Louisiana and Mississippi) required MCOs to make these changes for all types of Medicaid providers. States were also asked if their MCO contracts mandate minimum provider reimbursement rates. Of the 39 MCO states, ten indicated that they had no rate floors, 24 states indicated that they had rate floors for some provider types, and five states said they had minimum MCO payment requirements for all Medicaid provider types.
Provider Taxes and Fees
Provider taxes are an integral source of Medicaid financing. In this year’s survey, states reported continuing or increased reliance on provider taxes and fees to fund a portion of the non-federal share of Medicaid costs in FY 2017 and FY 2018. At the beginning of FY 2003, 21 states had at least one provider tax in place. Over the next decade, a majority of states imposed new taxes or fees and increased existing tax rates and fees to raise revenue to support Medicaid. By FY 2013, all but one state (Alaska) had at least one provider tax or fee in place.91 In FY 2017, 34 states had three or more provider taxes in place (Figure 9).
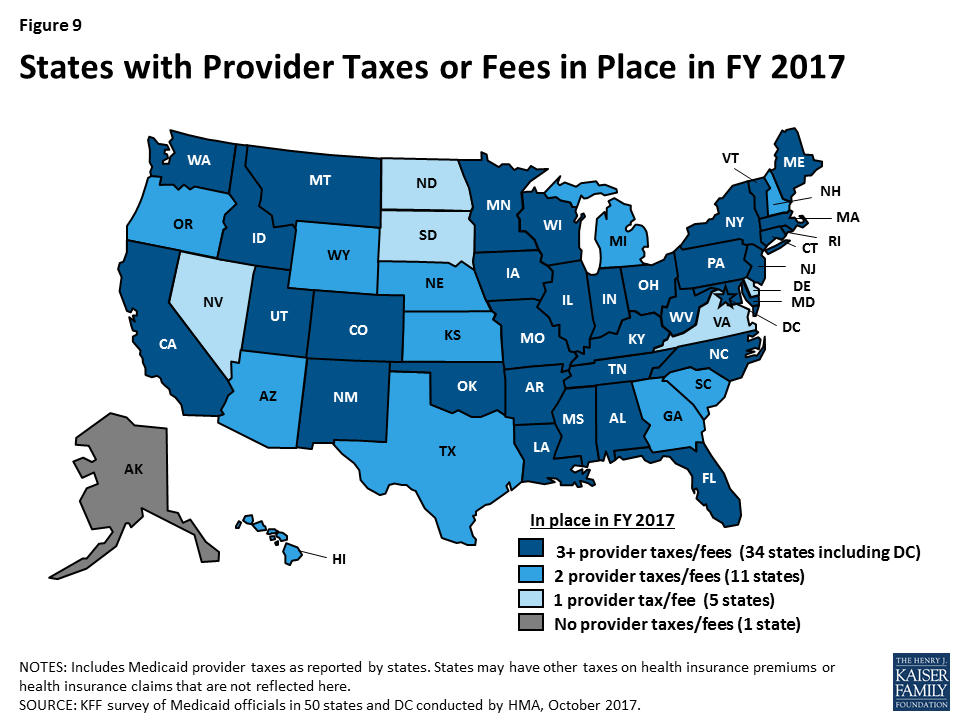
The most common Medicaid provider taxes in place in FY 2017 were taxes on nursing facilities (44 states), followed by taxes on hospitals (42 states) and taxes on intermediate care facilities for the intellectually disabled (36 states) (Table 14). Three states reported plans to add new taxes in FY 2018. Oregon reported a new MCO tax, Ohio’s MCO tax transitioned from a sales tax on premium revenues to a member month tax, and Tennessee expects to have a new ground ambulance provider assessment, which was enacted by the Tennessee General Assembly during its 2017 legislative session. Thirteen states reported increases to one or more provider taxes in FY 2018, compared to only five states reporting provider tax decreases.92
Recent federal health reform legislation93 under consideration in the Senate proposed phasing down the limit on state use of provider taxes (the “safe harbor threshold”) from the current allowable level, 6.0 percent of net patient revenues, to 5.0 percent of net patient revenues by FY 2025.94 Another proposal would lower the threshold to 4.0 percent in FY 2025.95 In this year’s budget survey, 29 states reported having at least one provider tax exceeding 5.5 percent of net patient revenues and 46 states reported having at least one provider tax exceeding 3.5 percent as of July 1, 2017. These data suggest that federal action to lower the safe harbor threshold would restrict states’ ability to supply the non-federal share to finance Medicaid and could therefore shift additional costs to states. If states were not able to find additional funds to replace provider tax funding, limits on provider taxes could result in program cuts with implications for Medicaid providers and beneficiaries.
TABLE 12: PROVIDER RATE CHANGES IN ALL 50 STATES AND DC, FY 2017
| States | Inpatient Hospital | Outpatient Hospital | Primary Care Physicians | Specialists | Dentists | Managed Care Organizations | Nursing Facilities | HCBS | Total | |||||||||
| Rate Change | + | – | + | – | + | – | + | – | + | – | + | – | + | – | + | – | + | – |
| Alabama | X | — | — | X | X | X | ||||||||||||
| Alaska | X | X | X | X | — | — | X | X | X | |||||||||
| Arizona | X | X | X | X | X | X | ||||||||||||
| Arkansas | X | — | — | X | X | X | ||||||||||||
| California | X | X | X | X | X | X | X | |||||||||||
| Colorado | X | X | X | X | X | |||||||||||||
| Connecticut | X | X | — | — | X | X | ||||||||||||
| Delaware | X | X | X | X | X | X | X | X | X | |||||||||
| DC | X | X | X | X | X | X | X | X | X | |||||||||
| Florida | X | X | X | X | X | X | X | |||||||||||
| Georgia | X | X | X | X | X | X | X | |||||||||||
| Hawaii | X | X | X | X | X | X | X | X | X | |||||||||
| Idaho | X | X | X | X | — | — | X | X | X | |||||||||
| Illinois | X | X | X | X | X | |||||||||||||
| Indiana | X | X | X | X | X | X | ||||||||||||
| Iowa | X | X | X | |||||||||||||||
| Kansas | X | X | X | X | X | X | X | X | X | X | ||||||||
| Kentucky | X | X | X | X | X | X | X | |||||||||||
| Louisiana | X | X | X | X | X | |||||||||||||
| Maine | X | — | — | X | X | X | ||||||||||||
| Maryland | X | X | X | X | X | X | ||||||||||||
| Massachusetts | X | X | X | X | X | X | ||||||||||||
| Michigan | X | X | X | X | X | X | ||||||||||||
| Minnesota | X | X | X | X | X | X | X | X | ||||||||||
| Mississippi | X | X | X | X | X | X | X | X | ||||||||||
| Missouri | X | X | X | X | X | X | X | X | X | X | ||||||||
| Montana | X | X | X | — | — | X | X | X | X | |||||||||
| Nebraska | X | X | X | X | X | X | X | X | ||||||||||
| Nevada | X | X | X | X | X | X | ||||||||||||
| New Hampshire | X | X | X | X | X | |||||||||||||
| New Jersey | X | X | X | X | X | X | X | X | ||||||||||
| New Mexico | X | X | X | X | X | X | X | X | X | |||||||||
| New York | X | X | X | X | X | X | ||||||||||||
| North Carolina | X | — | — | X | X | X | ||||||||||||
| North Dakota | X | X | X | X | X | X | X | |||||||||||
| Ohio | X | X | X | X | X | X | ||||||||||||
| Oklahoma | X | — | — | X | X | |||||||||||||
| Oregon | X | X | X | X | X | X | ||||||||||||
| Pennsylvania | X | X | X | X | X | |||||||||||||
| Rhode Island | X | X | X | X | X | X | ||||||||||||
| South Carolina | X | X | X | X | X | X | ||||||||||||
| South Dakota | X | X | X | X | X | — | — | X | X | |||||||||
| Tennessee | X | X | X | |||||||||||||||
| Texas | X | X | X | X | X | X | ||||||||||||
| Utah | X | X | X | X | X | X | X | |||||||||||
| Vermont | X | X | X | — | — | X | X | X | X | |||||||||
| Virginia | X | X | X | X | X | X | ||||||||||||
| Washington | X | X | X | X | X | |||||||||||||
| West Virginia | X | X | X | X | X | |||||||||||||
| Wisconsin | X | X | X | X | X | X | X | |||||||||||
| Wyoming | X | X | X | X | — | — | X | X | X | X | ||||||||
| Totals | 17 | 34 | 17 | 5 | 14 | 6 | 13 | 4 | 11 | 3 | 30 | 5 | 36 | 15 | 27 | 2 | 46 | 37 |
| NOTES: “+” refers to provider rate increases and “-” refers to provider rate restrictions. HCBS: Home and community-based services. For the purposes of this report, provider rate restrictions include cuts to rates for physicians, dentists, outpatient hospitals, managed care organizations, and HCBS as well as both cuts or freezes in rates for inpatient hospitals and nursing facilities. There are 12 states that did not have Medicaid MCOs in operation in FY 2017; they are denoted as ‘–‘ in the MCO column.SOURCE: Kaiser Family Foundation Survey of Medicaid Officials in 50 states and DC conducted by Health Management Associates, October 2017. | ||||||||||||||||||
TABLE 13: PROVIDER RATE CHANGES IN ALL 50 STATES AND DC, FY 2018
| States | Inpatient Hospital | Outpatient Hospital | Primary Care Physicians | Specialists | Dentists | Managed Care Organizations | Nursing Facilities | HCBS | Total | |||||||||
| Rate Change | + | – | + | – | + | – | + | – | + | – | + | – | + | – | + | – | + | – |
| Alabama | X | — | — | X | X | X | ||||||||||||
| Alaska | X | X | X | X | X | — | — | X | X | |||||||||
| Arizona | X | X | X | X | X | X | X | X | ||||||||||
| Arkansas | X | — | — | X | X | X | ||||||||||||
| California | X | X | X | X | X | X | X | X | X | |||||||||
| Colorado | X | X | X | X | X | X | X | X | ||||||||||
| Connecticut | X | X | — | — | X | X | ||||||||||||
| Delaware | X | X | X | X | X | X | X | X | X | |||||||||
| DC | X | X | X | X | X | X | ||||||||||||
| Florida | X | X | X | X | X | X | X | |||||||||||
| Georgia | X | X | X | X | X | X | X | X | X | |||||||||
| Hawaii | X | X | X | X | X | X | X | X | X | X | ||||||||
| Idaho | X | X | X | X | — | — | X | X | X | X | ||||||||
| Illinois | X | TBD | TBD | X | X | X | X | |||||||||||
| Indiana | X | X | X | X | X | |||||||||||||
| Iowa | X | X | TBD | TBD | X | X | X | |||||||||||
| Kansas | X | X | X | X | X | X | X | X | X | |||||||||
| Kentucky | X | X | X | X | X | X | ||||||||||||
| Louisiana | X | X | X | X | X | X | ||||||||||||
| Maine | X | — | — | X | X | X | X | |||||||||||
| Maryland | X | X | X | X | X | |||||||||||||
| Massachusetts | X | X | X | X | X | X | X | X | X | |||||||||
| Michigan | X | X | X | X | X | X | ||||||||||||
| Minnesota | X | X | X | X | X | X | X | |||||||||||
| Mississippi | X | X | X | X | X | X | ||||||||||||
| Missouri | X | X | X | X | X | X | X | X | X | X | ||||||||
| Montana | X | X | X | X | X | — | — | X | X | X | X | |||||||
| Nebraska | X | X | X | X | ||||||||||||||
| Nevada | X | X | X | X | X | X | X | X | X | |||||||||
| New Hampshire | X | X | X | X | X | X | ||||||||||||
| New Jersey | X | X | X | X | X | X | X | |||||||||||
| New Mexico | TBD | TBD | TBD | TBD | TBD | TBD | TBD | TBD | TBD | TBD | TBD | TBD | TBD | TBD | TBD | TBD | TBD | TBD |
| New York | X | X | X | X | X | X | ||||||||||||
| North Carolina | X | — | — | X | X | |||||||||||||
| North Dakota | X | X | X | X | X | |||||||||||||
| Ohio | X | X | TBD | TBD | TBD | TBD | TBD | TBD | X | X | TBD | TBD | X | X | ||||
| Oklahoma | X | — | — | X | X | |||||||||||||
| Oregon | X | X | X | X | X | X | X | |||||||||||
| Pennsylvania | X | X | X | X | ||||||||||||||
| Rhode Island | X | X | X | X | X | X | X | |||||||||||
| South Carolina | X | X | X | X | X | X | X | |||||||||||
| South Dakota | X | — | — | X | X | X | X | |||||||||||
| Tennessee | X | X | X | X | X | X | X | |||||||||||
| Texas | X | X | X | X | X | |||||||||||||
| Utah | X | X | X | X | X | X | ||||||||||||
| Vermont | X | X | X | — | — | X | X | X | X | |||||||||
| Virginia | X | X | X | X | X | X | ||||||||||||
| Washington | X | X | X | X | X | |||||||||||||
| West Virginia | X | X | X | X | X | |||||||||||||
| Wisconsin | X | X | TBD | TBD | TBD | TBD | TBD | TBD | TBD | TBD | X | X | ||||||
| Wyoming | X | — | — | X | X | X | X | |||||||||||
| Totals | 17 | 33 | 16 | 6 | 12 | 5 | 10 | 4 | 9 | 5 | 27 | 5 | 28 | 22 | 29 | 2 | 44 | 40 |
| NOTES: “+” refers to provider rate increases and “-” refers to provider rate restrictions. HCBS: Home and community-based services. For the purposes of this report, provider rate restrictions include cuts to rates for physicians, dentists, outpatient hospitals, managed care organizations, and HCBS as well as both cuts or freezes in rates for inpatient hospitals and nursing facilities. There are 12 states that did not have Medicaid MCOs in operation in FY 2017; they are denoted as “–” in the MCO column. TBD: At the time of the survey, calendar year 2018 MCO rates had not been set for Illinois, Iowa, or New Mexico. FY 2018 rates had not been determined for several categories of providers in Ohio and Wisconsin. New Mexico reported that rate decisions would be made “as needed” during FY 2018.SOURCE: Kaiser Family Foundation Survey of Medicaid Officials in 50 states and DC conducted by Health Management Associates, October 2017. | ||||||||||||||||||
TABLE 14: PROVIDER TAXES IN PLACE IN ALL 50 STATES AND DC, FY 2017 AND FY 2018
| States | Hospitals | Intermediate Care Facilities | Nursing Facilities | Other | ||||
| 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | |
| Alabama | X | X | X | X | X | X | ||
| Alaska | ||||||||
| Arizona | X | X | X | X | ||||
| Arkansas | X | X | X | X | X | X | ||
| California | X | X | X | X | X | X | X | X |
| Colorado | X | X | X | X | X | X | ||
| Connecticut | X | X | X | X | X | X | X | X |
| Delaware | X | X | ||||||
| DC | X | X | X | X | X | X | X | X |
| Florida | X | X | X | X | X | X | ||
| Georgia | X | X | X | X | ||||
| Hawaii | X | X | X | X | ||||
| Idaho | X | X | X | X | X | X | ||
| Illinois | X | X | X | X | X | X | ||
| Indiana | X | X | X | X | X | X | ||
| Iowa | X | X | X | X | X | X | ||
| Kansas | X | X | X | X | ||||
| Kentucky | X | X | X | X | X | X | X* | X* |
| Louisiana | X | X | X | X | X | X | X* | X* |
| Maine | X | X | X | X | X | X | X | X |
| Maryland | X | X | X | X | X | X | X | X |
| Massachusetts | X | X | X | X | X | X | ||
| Michigan | X | X | X | X | ||||
| Minnesota | X | X | X | X | X | X | X | X |
| Mississippi | X | X | X | X | X | X | X | X |
| Missouri | X | X | X | X | X | X | X* | X* |
| Montana | X | X | X | X | X | X | ||
| Nebraska | X | X | X | X | ||||
| Nevada | X | X | ||||||
| New Hampshire | X | X | X | X | ||||
| New Jersey | X | X | X | X | X | X | X* | X* |
| New Mexico | X* | X* | ||||||
| New York | X | X | X | X | X | X | X* | X* |
| North Carolina | X | X | X | X | X | X | ||
| North Dakota | X | X | ||||||
| Ohio | X | X | X | X | X | X | X | X |
| Oklahoma | X | X | X | X | X | X | ||
| Oregon | X | X | X | X | X | |||
| Pennsylvania | X | X | X | X | X | X | X* | X* |
| Rhode Island | X | X | X | X | X | X | ||
| South Carolina | X | X | X | X | ||||
| South Dakota | X | X | ||||||
| Tennessee | X | X | X | X | X | X | X | X* |
| Texas | X | X | X | X | ||||
| Utah | X | X | X | X | X | X | X | X |
| Vermont | X | X | X | X | X | X | X* | X* |
| Virginia | X | X | ||||||
| Washington | X | X | X | X | X | X | ||
| West Virginia | X | X | X | X | X | X | X* | X* |
| Wisconsin | X | X | X | X | X | X | X | X |
| Wyoming | X | X | X | X | ||||
| Totals | 42 | 42 | 36 | 36 | 44 | 44 | 24 | 25 |
| NOTES: This table includes Medicaid provider taxes as reported by states. Some states also have premium or claims taxes that apply to managed care organizations and other insurers. Since this type of tax is not considered a provider tax by CMS, these taxes are not counted as provider taxes in this report. (*) has been used to denote states with multiple “other” provider taxes.SOURCE: Kaiser Family Foundation Survey of Medicaid Officials in 50 states and DC conducted by Health Management Associates, October 2017. | ||||||||
Report: Benefits, Copayments, Pharmacy, And Opioid Strategies
Key Section Findings
A total of 26 states expanded or enhanced covered benefits in FY 2017 and 17 states plan to add or enhance benefits in FY 2018. The most common benefit enhancements reported were for behavioral health/substance use disorder services and dental services. Most states identified high cost and specialty drugs (including many states that specifically referenced hepatitis C antivirals) as a significant cost driver for state Medicaid programs. The majority (37 states in FY 2017 and 36 in FY 2018) reported actions to refine or enhance their pharmacy programs, especially implementation of new utilization controls (e.g., prior authorization requirements, clinical edits, quantity limits etc.). Thirty-five of 39 MCO states reported that the pharmacy benefit was “generally carved-in.” Of these 35 states, the majority reported requirements that MCOs have uniform clinical protocols (31 states) or uniform preferred drug lists (PDLs) (19 states) that will be in place for one or more drugs as of the end of FY 2018.
What to watch:
- A growing number of states have chosen to adopt the CDC guidelines for the prescribing of opioid pain medications for adults in primary care settings: 34 states reported adoption or plans for adoption in FY 2018 for their FFS programs (compared to 21 states in last year’s survey), and 18 states reported requiring MCOs to adopt the CDC guidelines or plans to do so in FY 2018 (compared to 11 in last year’s survey).
- Nearly all states have various FFS pharmacy management strategies targeted at opioid harm reduction including quantity limits (48 states); clinical criteria claim system edits (46 states); step therapy (34 states), and other prior authorization requirements (32 states). Somewhat fewer states (28) reported requirements in place for Medicaid prescribers to check their states’ Prescription Drug Monitoring Program before prescribing opioids to a Medicaid patient. For the 35 states that used MCOs to deliver pharmacy benefits, 24 reported that they required MCOs to follow some or all of their FFS pharmacy management policies for opioids.
- For FY 2017, the vast majority of states (46) reported that naloxone (a prescription opioid overdose antidote) was available in at least one formulation without prior authorization (PA) and most states (42) also covered the naloxone nasal spray formulation without PA. All 49 states that responded to a new question about medication-assisted treatment (MAT) drugs, reported coverage of buprenorphine and both oral and injectable naltrexone, but a somewhat smaller number (36 states) reported coverage of methadone in FY 2017.96
Tables 15 and 16 provide a complete listing of Medicaid benefit changes for FY 2017 and FY 2018. Table 17 provides a list of states that reported copayment actions for FY 2017 and 2018, and tables 18 and 19 provide additional details on Medicaid pharmacy benefit management strategies for opioids and naloxone coverage in FFS programs.
Benefit Changes
The number of states reporting new benefits and benefit enhancements continues to outpace the number of states reporting benefit cuts and restrictions. Twenty-six states reported new or enhanced benefits in FY 2017, and 17 states plan to add or enhance benefits in FY 2018. Fewer states reported benefit cuts or restrictions – six in FY 2017 and five in FY 2018 (Table 15 and Figure 10).
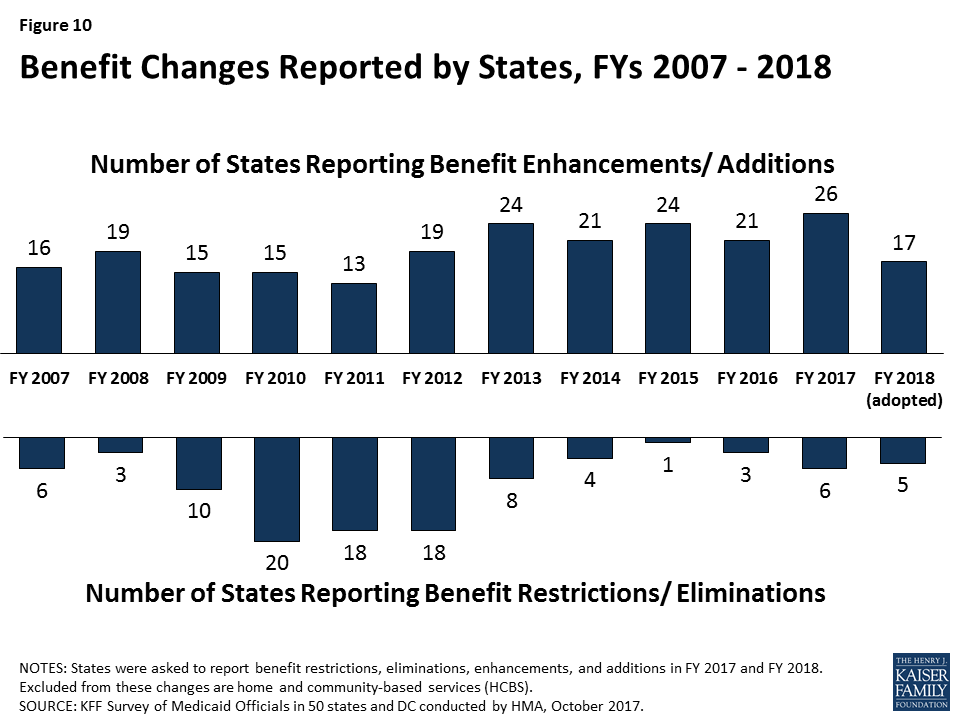
The most common benefit enhancements reported were for mental health and substance use disorder (SUD) services. Exhibit 12 also highlights states implementing other select benefit enhancements for dental, alternative pain therapies, and telemonitoring/telehealth.
| Exhibit 12: Select Categories of Benefit Enhancements or Additions | ||||
| Benefit | FY 2017 | FY 2018 | ||
| Mental Health/Substance Use Disorder Services | 9 States | IN, MA, NE, NH, NJ, RI, TX, VA, WI | 10 States | CO, HI, IN, MD, NE, NH, OH, UT, VA, WV |
| Dental Services | 5 States | AZ, IN, MD, OR, VT | 3 States | AZ, CA, UT |
| Alternative Pain Therapies (e.g., Chiropractic and Acupuncture) | 4 States | CA, DE, OH, OR | 2 States | IN, OH |
| Telemonitoring/ Telehealth Services | 2 States | NE, RI | 1 State | MD |
Nearly half of states that reported expanded mental health and/or SUD services made the changes as part of a new, comprehensive package of services versus a more limited benefit change. States using Section 1115 authority for SUD enhancements include states responding to CMS guidance97 issued in 2015, which describes a new Section 1115 waiver opportunity that supports states’ ability to provide more effective care to Medicaid beneficiaries with an SUD, including the provision of treatment services not otherwise covered under Medicaid. In addition to the dental and telehealth services benefit enhancements noted in Exhibit 12 and Table 16, many states also reported broader initiatives to increase access to dental care/improve oral health outcomes and to increase access to telehealth. See the “Emerging Delivery System and Payment Reform” section of this report for details on these initiatives.
Other key benefit expansions include:
- Family planning: Oregon is taking many steps to expand access to family planning services. In FY 2017, it added coverage of pharmacist-prescribed oral contraceptives, becoming (according to the state) the first Medicaid program in the country to do so. In FY 2018, it will cover a one-year supply of birth control pills, and is adding coverage of pharmacist-administered contraceptives (e.g., NuvaRing and Depo-Provera). Additional states adding family planning benefits include New Mexico (adding coverage of long-acting reversible contraceptive services as a separately billable service at Federally Qualified Health Centers and Rural Health Centers in FY 2017) and Nevada (adding coverage for a one-year supply of birth control pills in FY 2018).
- Cancer screenings: Four states reported cancer screening-related benefit enhancements. Louisiana and South Dakota added coverage for genetic testing for BRCA98 breast cancer gene mutations in FY 2017, Virginia added coverage for lung cancer screening with low dose computed tomography without prior authorization in FY 2017, and New York added coverage for digital breast tomosynthesis screening services in FY 2018. Louisiana also expanded coverage of breast reconstruction surgery to the contralateral unaffected breast for beneficiaries diagnosed with breast cancer.
Benefit restrictions reflect the elimination of a covered benefit or the application of utilization controls for existing benefits. The most common services restricted were dental services (Connecticut, Nevada, and Wyoming) and non-emergency medical transportation services (Arkansas and Massachusetts); however, most benefit restrictions in FY 2017 or FY 2018 were narrowly targeted. Some states restricting benefits provided exceptions for beneficiaries with mental health conditions or substance use disorders (Massachusetts and Nevada). One additional notable benefit restriction is Utah’s proposal to eliminate Early and Periodic Screening, Diagnostic, and Treatment (EPSDT) coverage for individuals ages 19 to 20 in FY 2018, subject to CMS approval.99
Tables 15 and 16 provide state-level information on benefit changes in FY 2017 and FY 2018.
Copayments
Federal law limits cost-sharing for people with income below 100 percent FPL to “nominal” amounts (defined in federal regulations), with higher amounts allowed for beneficiaries at higher income levels. Certain groups are exempt from cost-sharing, including mandatory eligible children, pregnant women, most children and adults with disabilities, people residing in institutions, and people receiving hospice care. In addition, certain services are exempt from cost-sharing: emergency services, preventive services for children, pregnancy-related services, and family planning services. Also, total Medicaid premiums and cost-sharing for a family cannot exceed 5 percent of the family’s income on a quarterly or monthly basis.100
Most state Medicaid programs require beneficiary copayments, but to varying degrees. Thirteen states reported changes to copayment requirements in either FY 2017 or FY 2018. Details about state actions related to copayments can be found in Table 17 and key changes are described below.
Six states reported new or increased copayment requirements. Key changes include:
- Three states (Michigan, New Hampshire, and New Mexico) reported new or increased copayments for enrollees with income above 100 percent of the FPL. In New Mexico, this change also applies to working individuals with disabilities.
- Three states (Maine, New Mexico, and Utah) reported new or increased copayments for non-emergency use of a hospital emergency department (ED). (These changes are part of pending Section 1115 waiver requests in Maine101 and Utah.)
- Colorado, New Mexico, and Utah are adding or increasing pharmacy copayments. Colorado reported increased copayments for hospital outpatient services.
Seven states reported policies that eliminate or reduce a copayment requirement for some or all covered populations. Key changes include:
- Three states (Indiana, North Dakota, and Tennessee) are reducing or eliminating higher copayments for non-emergency use of the ED.
- Oregon eliminated copayments for preventive services, Utah decreased inpatient copayments, and Vermont exempted sexual assault-related services from copayments.
TABLE 15: BENEFIT CHANGES IN ALL 50 STATES AND DC, FY 2017 AND FY 2018
| States | FY 2017 | FY 2018 | ||
| Enhancements/ Additions | Restrictions/ Eliminations | Enhancements/ Additions | Restrictions/Eliminations | |
| Alabama | ||||
| Alaska | ||||
| Arizona | X | X | ||
| Arkansas | X | |||
| California | X | X | ||
| Colorado | X | |||
| Connecticut | X | X | ||
| Delaware | X | |||
| DC | X | |||
| Florida | ||||
| Georgia | ||||
| Hawaii | X | |||
| Idaho | ||||
| Illinois | ||||
| Indiana | X | X | ||
| Iowa | ||||
| Kansas | ||||
| Kentucky | ||||
| Louisiana | X | X | ||
| Maine | ||||
| Maryland | X | X | ||
| Massachusetts | X | X | X | |
| Michigan | X | |||
| Minnesota | X | |||
| Mississippi | ||||
| Missouri | ||||
| Montana | ||||
| Nebraska | X | X | ||
| Nevada | X | X | X | X |
| New Hampshire | X | X | ||
| New Jersey | X | |||
| New Mexico | X | |||
| New York | X | |||
| North Carolina | ||||
| North Dakota | ||||
| Ohio | X | X | ||
| Oklahoma | X | X | X | |
| Oregon | X | X | ||
| Pennsylvania | ||||
| Rhode Island | X | |||
| South Carolina | ||||
| South Dakota | X | |||
| Tennessee | X | |||
| Texas | X | |||
| Utah | X | X | ||
| Vermont | X | |||
| Virginia | X | X | ||
| Washington | ||||
| West Virginia | X | X | ||
| Wisconsin | X | |||
| Wyoming | X | X | ||
| Totals | 26 | 6 | 17 | 5 |
| NOTES: States were asked to report benefit restrictions, eliminations, enhancements, and additions in FY 2017 and FY 2018. Home and community-based services (HCBS) and pharmacy benefit changes are excluded from this table.SOURCE: Kaiser Family Foundation Survey of Medicaid Officials in 50 states and DC conducted by Health Management Associates, October 2017. | ||||
Table 16: States Reporting Benefit Actions Taken in FY 2017 and FY 2018[endnote 239102-31]
| State | Fiscal Year | Benefit Changes |
| Arizona | 2017 | Adults (+): Add coverage for podiatry services (August 6, 2016). LTSS Adults (+): Add a $1,000 per year dental benefit for MLTSS beneficiaries (October 1, 2016). |
| 2018 | Adults (+): Add a $1,000 per year benefit for emergency dental services (October 1, 2017). Adults (+): Add coverage of outpatient occupational therapy services (October 1, 2017). | |
| Arkansas | 2017 | Expansion Adults (-): Eliminate non-emergency medical transportation coverage for expansion adults participating in Employer Sponsored Insurance feature of the Section 1115 waiver renewal (January 1, 2017). |
| California | 2017 | All (+): Restore acupuncture services (eliminated in 2009 for most populations excluding children, pregnant women, and nursing facility residents) (July 1, 2016). Pregnant Women (+): Add licensed midwives to the Comprehensive Perinatal Services Program (July 1, 2016). |
| 2018 | All (nc): Reaffirm coverage of non-emergency medical transportation as provided in state law (July 1, 2017). Adults (+): Fully restore coverage for dental services (January 1, 2018). | |
| Colorado | 2018 | Pregnant Women (+): Add coverage of up to three post-partum depression screenings in the first year following a child’s birth (July 1, 2017). Adults (+): Add coverage for Physical Therapy/Occupational Therapy services above the 12-hour cap with prior authorization (November 1, 2017). Children (+): Restore coverage of routine circumcisions as an elective benefit. |
| Connecticut | 2017 | Children (-): Apply additional restrictions on coverage of sealants and filling restorations (September 1, 2016). |
| 2018 | Adults (-): Apply annual cap on coverage for dental services (pending passage of FY 2018 state budget). | |
| Delaware | 2017 | Non-expansion Beneficiaries (+): Add coverage of chiropractic services (July 1, 2017). |
| District of Columbia | 2017 | All (+): Add Health Home services (“My Health GPS”) for beneficiaries with three or more chronic conditions (July 1, 2017). |
| Hawaii | 2018 | Adults (+): Expand mental health and substance abuse benefits including addition of intensive case management and tenancy supports for beneficiaries classified as chronically homeless (upon CMS approval). |
| Indiana | 2017 | All (+): Add coverage of physician-administered fluoride varnish (January 1, 2017). All (+): Expand coverage of tobacco dependence treatment (January 1, 2017). |
| 2018 | Adults (+): Add coverage of chiropractic spinal manipulation for HIP Plus enrollees (February 1, 2018). All (+): Add coverage of new substance use disorder treatment services, including expanded inpatient detoxification, additional residential services, addiction-specific outpatient treatment services, peer recovery supports, and relapse prevention (February 1, 2018). Adults (+): Increase member incentives for healthy behaviors to $200 per initiative, with a total of no more than $300 annually for HIP Basic and HIP Plus enrollees (February 1, 2018). | |
| Kansas | 2017 | Children (nc): Expand Autism-related services by moving three services from HCBS waiver coverage to State Plan coverage (January 1, 2017). |
| Kentucky | 2018 | All (nc): Expand non-emergency medical transportation services to include travel to pharmacies (July 1, 2017). |
| Louisiana | 2017 | Pregnant Women (+): Add coverage of mosquito repellant, when prescribed by a physician, for pregnant women and women trying to conceive as a Zika virus prevention measure (July 1, 2016). All (+): Add coverage of genetic testing for BRCA 1 and BRCA 2 breast cancer gene mutations (July 1, 2016). All (+): Expand coverage of breast reconstruction surgery to the contralateral unaffected breast for beneficiaries diagnosed with breast cancer (October 1, 2016). |
| 2018 | All (+): Expand coverage of preventive services eligible for the one percent increase in federal match under Section 4106 of the ACA (May 1, 2017). | |
| Maryland | 2017 | Children (nc): Add coverage for Applied Behavioral Analysis services for children with autism spectrum disorder to meet federal requirements (January 1, 2017). Foster Children (+): Expand coverage for dental services for former foster children up to age 26 (January 1, 2017). |
| 2018 | Adults (+): Add substance use disorder residential treatment services (July 1, 2017). All (+): Add coverage of remote patient monitoring for beneficiaries who meet qualifying medical criteria (January 1, 2018). | |
| Massachusetts | 2017 | All (+): Expand coverage of substance use disorder treatment services to include residential rehabilitation services and transitional support services (November 4, 2016). |
| 2018 | Medicaid Expansion (-): Eliminate coverage of non-emergency transportation services, except for transportation to substance use disorder treatment services for Medicaid expansion beneficiaries (November 1, 2017). All (+): Add coverage of enhanced “flexible services” as an incentive for beneficiaries to enroll in an ACO (March 1, 2018). | |
| Michigan | 2017 | Non-expansion Adults (+): Add coverage of preventive services assigned a grade A or B by the United States Preventive Services Task Force (USPSTF) (July 1, 2017). |
| Minnesota | 2017 | All (+): Add coverage of kidney transplants under Emergency Medical Assistance to eligible beneficiaries who are currently receiving dialysis services (July 1, 2016). All (+): Add coverage of gender confirmation surgery (January 1, 2017). All (+): Add coverage of community emergency medical technician services after discharge from a hospital or nursing home, and for safe home checks (January 1, 2017). |
| Nebraska | 2017 | Children (+): Add coverage for Multisystemic Therapy/Family Functional Therapy (July 1, 2016). All (+): Add coverage of telehealth services, including telemonitoring and originating site fee (January 1, 2017). |
| 2018 | All (+): Add coverage of nutrition services (July 1, 2017). All (+): Add coverage of peer support services (July 1, 2017). | |
| Nevada | 2017 | All (-): Reduce coverage of targeted case management services, to 10 hours in the initial month and five hours in the following three consecutive calendar months for adults without serious mental illness and children without serious emotional disturbance (February 23, 2017). All (+): Added coverage for paramedicine services (July 1, 2016). |
| 2018 | Non-expansion Beneficiaries (+): Add coverage of podiatry services (January 1, 2018). Non-expansion Beneficiaries (+): Add coverage of registered dietician services (July 1, 2017). Non-expansion Beneficiaries (+): Add coverage of home health durable medical equipment services (July 1, 2017). Non-expansion Beneficiaries (+): Add coverage of gender dysphoria services (January 1, 2018). Non-expansion Beneficiaries (-): Limit coverage of private duty nursing services and hospice services (July 1, 2017). Non-expansion Beneficiaries (-): Limit coverage of orthodontia services (July 1, 2017). Non-expansion Beneficiaries (-): Limit coverage of case management services with additional prior authorization requirements (July 1, 2017). All (+): Add coverage of one-year supply of birth control pills (July 1, 2017). | |
| New Hampshire | 2017 | Non-expansion Adults (+): Expand coverage of substance use disorder treatment services, to include assessment, outpatient services, residential treatment, opioid treatment programs, recovery support services, and recovery monitoring (July 1, 2016). |
| 2018 | Children (+): Expand coverage of behavioral health services for children with serious emotional disturbance (January 1, 2018). | |
| New Jersey | 2017 | Non-expansion Adults (+): Expand substance use disorder benefit to align with the state’s Alternative Benefit Package for Medicaid expansion beneficiaries (July 1, 2016). |
| New Mexico | 2017 | All (+): Add coverage of long-acting reversible contraception services as a separately billable service at FQHCs and RHCs (September 1, 2016). All (+): Add coverage of medication monitoring services by nurses and physician assistants (January 1, 2017). Pregnant Women (nc): Add coverage of licensed birthing centers as a new provider type to meet federal requirements (February 25, 2017). |
| New York | 2018 | Children (+): Add coverage of pasteurized donor human breast milk for infants <1500 grams (April 1, 2017). All (+): Add coverage of continuous glucose monitoring devices for beneficiaries with Type 1 diabetes (September 1, 2017). All (+): Add coverage of digital breast tomosynthesis (DBT) screening services (September 1, 2017 for FFS and November 1, 2017 for managed care). All (+): Add limited infertility benefit for women between the ages of 21 to 44 (September 1, 2017, pending CMS approval). |
| North Dakota | 2017 | Children (nc): Add coverage for Applied Behavioral Analysis services for children with autism spectrum disorder to meet federal requirements (June 1, 2017). |
| Ohio | 2017 | All (+): Add coverage of acupuncture services (January 1, 2017). |
| 2018 | All (+): Expand coverage of behavioral health services to include assertive community treatment for adults, family counseling, intensive home-based treatment for youth at risk of out-of-home placement, and primary care services delivered by a behavioral health provider (January 1, 2018). All (+): Expand provider types who may provide acupuncture services (October 1, 2017). | |
| Oklahoma | 2017 | Children (+): Mandate polycarbonate lenses for children (September 1, 2016). Pregnant Women (-): Limit high-risk obstetrical services, such as quantity limits on ultrasounds (September 1, 2016). All (+): Provide coverage of non-emergency medical transportation services for additional passengers (October 1, 2016). Children (+): Remove barriers to receiving school-based services for children with IEPs (November 1, 2016). |
| 2018 | Adults (-): Eliminate coverage of non-mandatory over-the-counter drugs (October 1, 2017). | |
| Oregon | 2017 | Adults (+): Restore previously cut restorative dental benefits (relaxed limitation criteria for dentures; coverage for crowns; scaling and planning) (July 1, 2016). Adults (+): Expand coverage for alternative back pain therapies including acupuncture, chiropractic manipulation, and yoga (July 1, 2016). Children (nc): Added coverage for Applied Behavioral Analysis services for children with autism spectrum disorder to meet federal requirements (July 1, 2016). All (+): Add coverage of pharmacist-prescribed oral contraceptives, as permitted under state law (January 1, 2017). |
| 2018 | All (+): Add coverage of one-year supply of birth control pills and pharmacist-administered contraceptives (i.e., NuvaRing and Depo-Provera) (January 1, 2018). All (+): Add coverage of other pharmacist-prescribed medications (TBD), as permitted under state law (January 1, 2018). | |
| Rhode Island | 2017 | All (+): Add coverage for home stabilization services. All (+): Add coverage for telehealth services in new managed care contracts. Adults (+): Implement the Sobering Treatment Opportunity Program (STOP), an ER diversion pilot in Providence that will cover an overnight stay and referral to appropriate counseling for beneficiaries with chronic alcohol dependence who are homeless. |
| South Carolina | 2018 | Children (nc): Add autism spectrum disorder services to the State Plan for eligible beneficiaries up to age 21 to meet federal requirements (July 1, 2017). |
| South Dakota | 2017 | Adults (+): Add coverage of genetic testing for BRCA breast cancer gene mutations (July 1, 2016). |
| Tennessee | 2017 | Adults (-): Limit Allergy Immunotherapy to clinical practice guidelines (July 1, 2016). |
| Texas | 2017 | Children (+): Add coverage for family therapy without the patient present as a benefit for children under age 21 (January 1, 2017). Children (+): Add coverage for Prescribed Pediatric Extended Care Centers for medically fragile children receiving extensive private duty nursing services at home, up to 12 hours (July 1, 2017). |
| Utah | 2018 | All (+): Add coverage of screening, brief intervention, and referral to treatment services (July 1, 2017). Aged, Blind or Disabled (+): Add coverage of dental services for the blind and disabled (July 1, 2017). Adults (-): Eliminate EPSDT coverage for parents and childless adults age 19 to 20 (effective the later of January 1, 2018, or upon CMS approval). |
| Vermont | 2017 | All (+): Allow licensed dental hygienists to bill Medicaid directly (July 1, 2016). |
| Virginia | 2017 | All (+): Expand coverage of addition recovery treatment services, including residential treatment, day treatment/partial hospitalization, intensive outpatient treatment, medication-assisted treatment, substance use case management, inpatient detoxification, inpatient substance use disorder treatment, and residential or inpatient substance use disorder treatment in an institution of mental disease with greater than 16 beds (April 1, 2017). All (+): Add coverage of lung cancer screening with low dose computed tomography without prior authorization (January 1, 2017). |
| 2018 | Traditional Beneficiaries (+): Add coverage for peer support services for beneficiaries with serious mental illnesses and/or substance use disorders (July 1, 2017). Limited Adult Coverage Group (+): Expand coverage of addiction recovery and treatment services and add coverage for peer support services for beneficiaries with serious mental illnesses and/or substance use disorders (October 1, 2017). | |
| West Virginia | 2017 | All (+): Expand coverage of Hepatitis C antiviral agents with a revised clinical policy (April 1, 2017). |
| 2018 | All (+): Expand coverage of substance use disorder services, including services provided by institutions for mental disease, peer recovery support services, and Naloxone treatment (January 1, 2018). | |
| Wisconsin | 2017 | All (+): Add licensed midwives as an allowable Medicaid provider (January 1, 2017). All (+): Add coverage of residential substance abuse treatment through comprehensive community service programs (May 1, 2017). |
| Wyoming | 2017 | All (+): Add coverage for dietician services (July 1, 2016). Aged, Blind and Disabled (-): Reduce nursing facility bed-hold days (October 1, 2016). Aged, Blind and Disabled (-): Limit behavioral health, therapy, and home health services by imposing soft caps (January 1, 2017). Adults (-): Eliminate coverage of dental services. |
Table 17: Copayment Actions Taken in the 50 States and DC, FY 2017 and FY 2018[endnote 239102-32]
| State | Fiscal Year | Copayment Changes |
| Colorado | 2018 | Increase: Increase pharmacy copayment to $3.00 per prescription for all non-exempt eligibility groups (1/1/2018). Increase: Double the hospital outpatient copayments for all non-exempt eligibility groups (1/1/2018). |
| Delaware | 2018 | Decrease: Treatment of pre-eligibility medical expenses in determining post eligibility cost of care contribution for LTSS population; “look-back” period expanded from 30 days to 90 days. Potential to reduce the monthly “patient pay” amount (effective date dependent on SPA approval). |
| Indiana | 2018 | Decrease (for HIP 2.0 expansion population): Eliminating the graduated copayment for non-emergent ER use (2/1/2018). |
| Maine | 2018 | New: Maine’s Section 1115 waiver would impose a copay on all populations for non-emergent use of the ED. (Dual eligibles, those in institutions and a few other groups are exempt.) |
| Michigan | 2017 | Increase (for adult enrollees with incomes between 100% and 133% FPL): Increase in prescription, hospital, and office visit copays. Copays were doubled (4/1/2017). |
| New Hampshire | 2018 | Increase (for adult enrollees with incomes between 100% and 133% FPL): Increase in Copayments for Inpatient Hospital, Primary and BH Care, Imaging, X-rays, and PT/OT Services (1/1/2018). |
| New Mexico | 2018 | New (for most populations): Copays for brand-name prescriptions when there is a less expensive generic equivalent medicine available (1/1/2018). New (for most populations): Copays for non-emergency use of the emergency department (1/1/2018). New (for Working Disabled, Adult expansion group with income above 100% FPL): New copayments for outpatient office visits (excluding behavioral health), inpatient stays, outpatient surgeries, and pharmacy (1/1/2018). |
| North Dakota | 2017 | Elimination (for all Medicaid groups): Higher copayment for non-emergency use of the ER was eliminated (1/1/2017). |
| Oregon | 2017 | Elimination (for all Medicaid groups): Copayments were eliminated for preventive services, office visits, and pharmacy (1/1/2017). |
| Tennessee | 2017 | Decrease (for waiver-eligible children): Copayment for non-emergency use of the ER was reduced from $10 to $8.20 (12/16/2016). |
| Utah | 2018 | Decrease: Inpatient copayments will be reduced to comply with federal maximum (date TBD). Increase: Outpatient copayments are being increased for all but children and pregnant women (date TBD). Increase (for current enrollees and childless adults): Establish a $25 copay for non-emergency use of the ER (1/1/18). Increase: Increase pharmacy copayments. |
| Vermont | 2017 | Decrease: Remove copays for sexual assault-related services for all Medicaid groups (10/1/2016). |
| West Virginia | 2018 | Neutral: Changing from a tiered copayment based on cost to $1 generic and $3 brand (date TBD). |
Prescription Drug Utilization and Cost Control Initiatives
Almost all states have implemented aggressive strategies to slow Medicaid spending growth for prescription drugs, including preferred drug lists (PDLs), supplemental rebate programs, and state maximum allowable cost programs. In recent years, however, a disproportionate increase in prescription drug costs relative to overall spending has heightened state attention on pharmacy reimbursement and coverage policies. In this year’s survey, states reported a variety of actions in FY 2017 and FY 2018 to refine and enhance their pharmacy programs, including actions to react to new and emerging specialty and high-cost drug therapies.
Pharmacy Cost Drivers
This year’s survey asked states to identify the biggest cost drivers that affected growth in total pharmacy spending102 (federal and state) in FY 2017 and projected for FY 2018. Consistent with the results of the 2015 and 2016 surveys, most states identified specialty and high cost drugs as the most significant cost driver, with many states pointing specifically to hepatitis C antivirals. For these drugs, high costs are attributable to the high per prescription cost as well as increased utilization. Two states (Georgia and Tennessee) noted that they are seeing hepatitis C antiviral costs moderating (although costs remain high compared to other drugs). Other specialty drugs, behavioral health, and/or substance use disorder drugs were cited as cost drivers, and some specific drug classes (such as hemophilia factor, oncology drugs, and diabetes products) were also identified as major cost drivers. For FY 2018, several states also cited a new spinal muscular atrophy drug (Spinraza), priced at $125,000 a dose, or $750,000 for the first year and $375,000 per year thereafter for life (due to fewer doses per year).
Medicaid Covered Outpatient Drug Final Rule
State Medicaid programs historically reimbursed pharmacies for the “ingredient cost” of each prescription using an Estimated Acquisition Cost (EAC), plus a dispensing fee.103 The new federal Covered Outpatient Drug final rule104 replaced EAC with “Actual Acquisition Cost” (AAC) and required states to provide a “professional dispensing fee” that reflects the pharmacist’s professional services and costs to dispense a drug to a Medicaid beneficiary. States can determine their own AAC prices or use the pricing files published and updated weekly by CMS – the “National Average Drug Acquisition Costs” (NADACs) – which are derived from outpatient drug acquisition cost surveys of retail community pharmacies.105 Some states had already transitioned to an AAC methodology prior to the issuance of the final rule, but all other states were required to come into compliance by April 1, 2017. The new methodology generally results in lower ingredient cost reimbursement but higher dispensing fees.–This year’s survey asked states whether implementation of the rule’s AAC and professional dispensing fee requirements was expected to result in budget savings or greater costs, or be budget neutral. Most states reported that implementation of the rule was expected to have a budget neutral impact (16 states) or result in savings (12 states). Fourteen states reported an expectation of greater costs.106 Several states reported adoption of an AAC methodology prior to FY 2017 (8 states), and one of these states (Idaho) commented that it was continuing to achieve savings as a result of Medicaid provider rate changes and provider taxes and fees in place in FY 2017 and FY 2018. For purposes of this report, implementation of the Covered Outpatient Drug final rule is not counted as a cost containment action because it is an implementation of a federal regulatory requirement.
Pharmacy Cost Containment Actions in FY 2017 and FY 2018
Almost all states had prescription drug cost containment policies (including prior authorization requirements and PDLs) in place prior to FY 2017, and most are constantly refining and updating these policies. Although states may not have reported every refinement or routine change in this year’s survey, 37 states in FY 2017 and 36 states in FY 2018 reported implementing or making changes to a wide variety of cost containment initiatives in the area of prescription drugs, comparable to the number of states taking such actions in recent years. By far the most frequently cited action was the application of new or expanded utilization controls (e.g., prior authorization requirements, clinical edits, and quantity limits) reported by 32 states in FY 2017 and 29 in FY 2018. Sixteen states in FY 2017 and 17 in FY 2018 also reported new or expanded initiatives to generate greater rebate revenue, including New York which is implementing a new state law in FY 2018 that applies a cap on Medicaid drug expenditures as a separate component of the global state Medicaid spending cap that the state has had in place since 2011. If the state determines that drug spending will exceed the annual growth limit, the Commissioner of the Department of Health may identify and refer drugs to the Drug Utilization Review (DUR) Board for a recommended target supplemental rebate.
Other frequently cited newly implemented or expanded pharmacy cost containment actions were:
- Provider education or profiling initiatives (14 states in FY 2017 and 16 in FY 2018)
- Initiatives to reduce pharmacy-related fraud, waste, and abuse (13 states in FY 2017 and 14 in FY 2018)
- Medication therapy management programs (8 states in FY 2017 and 7 in FY 2018)
Managed Care’s Role in Delivering Pharmacy Benefits
Since the passage of the ACA, states have been able to collect rebates on prescriptions purchased by managed care organizations (MCOs) operating under capitated arrangements. As a result, many states have chosen to “carve in” the pharmacy benefit to their managed care benefits. As more states have enrolled additional Medicaid populations into managed care arrangements over time, and as Medicaid enrollment has increased due to ACA coverage expansions, MCOs have played an increasingly large role in administering the Medicaid pharmacy benefit. In this year’s survey, states with MCO contracts were asked whether pharmacy benefits were covered under those contracts as of July 1, 2017.
Of the 39 states contracting with comprehensive risk-based MCOs, 35 states reported that the pharmacy benefit was “generally carved-in (with possible exceptions)” including Nebraska that completed a full pharmacy carve-in during FY 2017 and Indiana that completed a pharmacy carve-in for its Hoosier Healthwise program (for low-income pregnant women and children) in FY 2017. Among the states that carved drugs into MCOs, several reported carve-outs for selected drug classes. The most common drugs carved out were behavioral health drugs and HIV drugs (California, Maryland, and Michigan), hemophilia clotting factor (California, Florida, Michigan, and New Hampshire), and hepatitis C antivirals (Colorado, Massachusetts, Michigan, New Hampshire, South Carolina, and Texas). New York reported, however, that it reversed its carve-out of hemophilia clotting factor in July 2017.
Four states (Missouri, Tennessee, West Virginia, and Wisconsin) reported that the pharmacy benefit was “generally carved-out,” including West Virginia that completed a full carve-out as of July 2017. While Wisconsin noted that pharmacy was carved into its Family Care Partnership program (an integrated health and long-term care program for frail elderly and people with disabilities), the state noted that this program had a very small enrollment (approximately 3,000 as of July 2017107 ) and that all other Wisconsin Medicaid enrollees received their pharmacy benefit through the FFS delivery system.
Prior reports show that nearly all states use prior authorization and PDLs in FFS programs. This year’s survey asked whether MCOs were required (in FY 2017) or would be required (in FY 2018) to adhere to uniform clinical protocols (state prescribed medical necessity criteria) for one or more drugs or a uniform PDL (state prescribed requirements for designating a specified drug product as either preferred, meaning covered without the need to obtain prior authorization, or non-preferred). This means that to the extent states impose these policies in FFS, the same policies would apply in managed care. Compared to last year’s survey, there was a notable increase in the number of states with uniform clinical protocols in place and a modest increase in the number of states that reported having a uniform PDL requirement in place. States were also asked whether MCO contracts included risk-sharing provisions for one or more drugs (e.g., risk corridors, risk pools, reinsurance, etc.), a new question not included in last year’s survey (Exhibit 13).
| Exhibit 13: Managed Care Pharmacy Policies | |||||||
| Policy | In Place in FY 2017 | FY 2018 Changes | |||||
| New | Expanded | ||||||
| Uniform Clinical Protocols(1 or more drugs) | 28 States | CA, DC, DE, GA, HI, IA, IL, IN, KS, KY, MA, MD, MI, MS, NE, NJ, NM, NV, NY, OH, OR, PA, RI, TX, UT, VA, WA, WV* | 3 States | LA, ND, VA | 7 States | DE, KY, MA, NV, OH, PA, WA | |
| Uniform PDL(1 or more drug classes) | 15 States | AZ, DE, FL, IA, KS, LA, MN, MS, NE, NV, OR, TX, UT, WA, WV* | 4 States | IL, ND, OH, VA | 2 States | LA, WA | |
| Risk-sharing (for 1 or more drugs) | 15 States | AZ, CA, DE, FL*, HI, IN, KS, MA, NM, NV, OH, OR, PA, RI, VA | 0 States | 5 States | DE, HI, IN, MA, SC | ||
| * WV removed the pharmacy benefit from its MCO contracts July 1, 2017. FL discontinued hepatitis C kick-payments in August 2017. | |||||||
Uniform clinical protocols and PDL requirements reported by states were often limited to one or a few specific drug classes. Hepatitis C antivirals were the most commonly mentioned drug class targeted by uniform clinical protocols (reported by DC, Georgia, Hawaii, Illinois, Maryland, New Jersey, New Mexico, Oregon, Rhode Island, Pennsylvania, and Virginia) and were also reported as a specific focus of uniform PDL requirements in Minnesota and Oregon. Strategies reported by states to mitigate or share financial risk with MCOs for certain high cost drugs included selected drug carve-outs, risk corridors, kick payments,108 and risk pools, and were most commonly applied to hepatitis C antivirals, but in some cases were applied to drugs above a certain dollar threshold (Hawaii), cystic fibrosis drugs (Pennsylvania), and hemophilia clotting factor (Delaware). Two states also commented that new risk-sharing arrangements were currently under consideration for a new spinal muscular atrophy drug and one state was considering a risk sharing arrangement for a Duchenne muscular dystrophy drug.
Opioid Harm Reduction Strategies
According to the Centers for Disease Control and Prevention (CDC), drug overdose deaths continue to increase in the United States and the majority of these deaths (six out of ten) involve an opioid (including prescription opioids and heroin).109 The CDC cites the amount of prescription opioids sold in the United States – which have nearly quadrupled since 1999 – as a driving factor in opioid overdose deaths, which have more than quadrupled since 1999.110 Medicaid plays an important role in addressing the epidemic, covering 3 in 10 people with opioid addiction in 2015 and facilitating access to a number of addiction treatment services.111 In a January 2016 Informational Bulletin,112 CMS highlighted the important role state Medicaid programs can play to help address the opioid epidemic in their states by encouraging safer opioid alternatives for pain relief, working with other state agencies to educate Medicaid providers on best practices for opioid prescribing, employing pharmacy management practices (PDL placement, clinical criteria, prior authorization, quantity limits, etc.) and working to increase access to naloxone, an overdose antidote. In this year’s survey, we asked states about their pharmacy benefit strategies for preventing opioid harm in place in FY 2017 and planned for FY 2018 and their coverage of certain medication-assisted treatment (MAT) medications.
CDC Opioid Prescribing Guidelines
This year’s survey shows a growing number of states choosing to adopt the CDC guidelines for the prescribing of opioid pain medications for adults in primary care settings.113 Both last year and this year, the survey asked states if their Medicaid program has adopted or is planning to adopt these guidelines in their FFS programs or as a requirement for MCOs to adopt. As shown in Exhibit 14 below, 34 states reported adoption or plans for adoption in FY 2018 for their FFS programs (compared to 21 states in last year’s survey). Of the 39 states with MCO contracts, 18 states reported requiring MCOs to adopt the CDC guidelines or plans to do so in FY 2018 (compared to 11 states in last year’s survey). Many other states indicated that these policies were under review for FFS and MCOs.
| Exhibit 14: Number of States Adopting CDC Opioid Prescribing Guidelines | ||||
| Status | For FFS | As a requirement for MCOs to adopt | ||
| Yes, have adopted | 23 States | AR, AZ, CT,* FL, ID, IN, KY, LA, MA, ME, MS, NE, NH, NV, NY, OR, PA, TN, TX,* VA, VT, WA,* WV | 8 States | IN, KY, LA, MS, NE, NH, VA, WA, |
| Plan to adopt in FY 2018 | 11 States | DC, GA, IA, KS, MD, MN, MO, MT, NC, NM, SC | 10 States | DC, DE, IA, KS, MA, MD, MN, NV, PA, SC |
| * CT and TX reported adoption of part, but not all, of the CDC guidelines. WA indicated that its state statutory prescribing guidelines include a Morphine Equivalent Dose (MED) limit (110 mg) that differs from the CDC limit (90 mg). | ||||
States were also asked to describe any implementation challenges related to the CDC guidelines. The most commonly reported challenge was the inability of a state’s claims processing system to apply the Morphine Equivalent Dose (MED) limit across multiple products and multiple prescription claims. Other reported challenges included obtaining stakeholder consensus and support (including providers); titrating dosages downward for patients who have been stabilized on higher dosages; and the inability to control or enforce appropriate prescribing behavior. One state also expressed concern that the CDC’s MED limit is too low and could have the unintended consequence of driving up heroin use and overdoses. A few states reported having state guidelines already in place that were aligned with the CDC guidelines.
Medicaid Pharmacy Benefit Management Strategies
The January 2016 CMS Informational Bulletin highlighted Medicaid pharmacy benefit management strategies for preventing opioid-related harms.114 The survey asked states to report strategies that were in place in FY 2017 for FFS and changes to these strategies planned for FY 2018. Specifically, the survey asked about the following strategies: opioid quantity limits,115 clinical criteria claim system edits116 (subject to prior authorization (PA) override), step therapy PA criteria,117 other PA requirements for opioids, and requirements that prescribers check the state’s Prescription Drug Monitoring Program (PDMP) before prescribing opioids.118 All but one state reported having at least one of these opioid-focused pharmacy management policies in FFS in place in FY 2017, and nearly three-quarters of states (37) plan to take at least one action in FY 2018 to newly implement or increase opioid controls through one of these strategies. See Exhibit 15 and Table 18 for details on states implementing or expanding these controls.
| Exhibit 15: States Implementing Opioid-Focused Pharmacy Benefit Management Strategies in FFS | |||
| Strategy | In Place in FY 2017(# of states) | FY 2018 (# of states) | |
| New | Expanded | ||
| Quantity Limits | 48 | 3 | 26 |
| Clinical criteria claim system edits (subject to Prior Authorization override) | 46 | 1 | 21 |
| Step Therapy PA criteria | 34 | 1 | 6 |
| Other Prior Authorization | 32 | 5 | 14 |
| Required use of Prescription Drug Monitoring Programs | 28 | 6 | 3 |
For states that use MCOs to deliver pharmacy benefits, the survey asked whether, as of July 1, 2017, the MCOs were required to follow the state’s FFS pharmacy benefit management policies for opioids. Of the 35 states with MCOs that deliver pharmacy benefits, 12 states responded “yes” and 12 states responded “yes, in part.” Of the 12 states answering “yes in part,” a few indicated that their MCOs were in the process of coming into alignment with the state’s FFS policies. One state indicated that MCOs may conduct additional maximum dose and quantity reviews; one state reported that MCOs must adopt the FFS management strategies but have some latitude in preferred drug selection and specific clinical criteria, and one state reported that MCOs may provide additional naloxone coverage without prior authorization and/or naloxone atomizers.
Other Pharmacy Management Strategies
A few states mentioned other pharmacy management strategies in use or planned including the following:
- Day limits were applied by Arizona and Utah (no more than seven days for the initial fill of any prescription opioid), Colorado (seven-day supply limit for opioid naïve patients), and Vermont (seven-day limit applied for adults and a three-day limit applied for children).
- MED limits were applied or lowered in Colorado, Maryland, and Vermont, and Connecticut implemented an “MME calculator” to provide prescribers with a MME (morphine milligram equivalent) calculation at the point of service.
- Maryland reported a new requirement for prescriber attestations (checked PDMP, drug urine test, offered naloxone, pain management contract).
- Nevada and DC reported pharmacy lock-in programs.119
- Oregon reported expanding access to medication-assisted treatment drug by ending its lock-in program for Suboxone.
Access to Naloxone
Naloxone is a prescription opioid overdose antidote that prevents or reverses the life-threatening effects of opioids including respiratory depression, sedation, and hypotension. There are three FDA-approved formulations of naloxone: an injectable formulation offered as a generic; a brand, prefilled auto-injection formulation (approved in 2014) designed for use by persons without medical training (Evzio); and a brand prepackaged nasal spray (approved in 2015) (Narcan).120 All formulations have experienced significant price increases in recent years; most notably, the list price for the auto-injection formulation increased from $690 in 2014 to $4,500 in 2016.121 In this year’s survey, states were asked whether the various naloxone formulations were available without a prior authorization (PA) in their Medicaid programs in FY 2017 and whether any changes were planned for FY 2018. States were also asked if naloxone coverage was provided for family members and friends obtaining prescriptions on an enrollee’s behalf. See Exhibit 16 and Table 19 for details on state naloxone pharmacy benefit management strategies.
A number of states commented that the auto-injector manufacturer (Evzio) had ended its participation in the federal drug rebate program for this product which allows states to eliminate all Medicaid coverage of this product (with or without PA). Of the 10 states reporting coverage of Evzio without PA in FY 2017, one state reported plans to move the Evzio to non-preferred status in FY 2018 (subject to PA) and three states indicated all coverage would be eliminated.
| Exhibit 16: States Implementing Naloxone Pharmacy Benefit Management Strategies in FFS | |||
| Strategy | In Place in FY 2017 (# of states) | FY 2018 (# of states) | |
| New | Expanded | ||
| Naloxone available in at least one formulation without prior authorization (PA) | 46 | 1 | 1 |
| Naloxone nasal spray covered without PA | 42 | 0 | 1 |
| Naloxone nasal spray atomizer covered without PA | 20 | 0 | 0 |
| Naloxone auto-injector covered without PA | 10* | 0 | 0 |
| Naloxone coverage provided for family members and friends obtaining prescriptions on enrollee’s behalf | 11 | 0 | 0 |
| * Three of these states (LA, MD, and MN) reported ending coverage of naloxone auto-injectors in FY 2018 and one state (NV) changed to non-preferred status. | |||
Medication-Assisted Treatment
The ACA requires state Medicaid programs to provide coverage for treating substance use disorders (SUDs) for their ACA expansion populations, but does not specify which SUD services must be included. This requirement has bolstered states’ work to respond to the opioid epidemic. The standard of care for opioid use disorder is medication-assisted treatment (MAT), which combines psychosocial treatment with medication.122 Compared to psychosocial treatment alone, MAT is associated with greater adherence to treatment, decreased opioid use, and reduced likelihood of overdose fatalities.123 The FDA has approved the following medications that can be used as part of MAT for opioid use disorder: methadone, buprenorphine, and both oral and extended-release injectable naltrexone.124 In this year’s survey, states were asked whether they covered each of these drugs (when used to treat opioid use disorders) or planned to add coverage in FY 2018. All 49 states that responded reported coverage of buprenorphine and both oral and injectable naltrexone, but a somewhat smaller number (36 states) reported coverage of methadone in FY 2017.125 However, one state reported plans to add coverage for methadone in FY 2018 (Indiana) and five states reported that methadone coverage was under consideration (Kentucky, Louisiana, North Dakota, South Carolina, and West Virginia). Seven states (Alabama, Idaho, Iowa, Nebraska, Tennessee, Texas, and Wyoming) reported no coverage or plans to add coverage for Methadone.
TABLE 18: MEDICAID FFS PHARMACY BENEFIT MANAGEMENT STRATEGIES FOR OPIOIDS IN ALL 50 STATES AND DC, IN PLACE IN FY 2017 AND ACTIONS TAKEN IN FY 2018
| States | Opioid Quantity Limits | Clinical Edits in Claim System | Opioid Step Therapy Requirements | Other Prior Authorization Requirements for Opioids | Required use of Prescription Drug Monitoring Programs | Any Opioid Management Strategies | ||||||
| In place FY 2017 | New/Exp FY 2018 | In place FY 2017 | New/Exp FY 2018 | In place FY 2017 | New/Exp FY 2018 | In place FY 2017 | New/Exp FY 2018 | In place FY 2017 | New/Exp FY 2018 | In place FY 2017 | New/Exp FY 2018 | |
| Alabama | X | X | X | X | X | X | ||||||
| Alaska | X | X | X* | X | X | |||||||
| Arizona | X | X | X | X | X | X | X | X | X | |||
| Arkansas | X | X | X | X | X | X | X | |||||
| California | X | X | X | X* | X | X | ||||||
| Colorado | X | X | X | X | X | |||||||
| Connecticut | X | X | X | X | ||||||||
| Delaware | X | X | X | X* | X | X | X | |||||
| DC | X | X | X | X* | X | X | X | |||||
| Florida | X | X | X | X | X | X | X* | X | X | |||
| Georgia | X | X | X | X | X | X | ||||||
| Hawaii | X* | X* | ||||||||||
| Idaho | X | X | X | X | X | X | X | X | ||||
| Illinois | X | X | X | X | X | |||||||
| Indiana | X | X | X | X | X | |||||||
| Iowa | X | X | X | X | X | X | X* | X | X | X | ||
| Kansas | X | X | X | X | X | X | X | X | ||||
| Kentucky | X | X | X | X | X | X | X | X | X | |||
| Louisiana | X | X | X | X | X | X | X | X | X | X | ||
| Maine | X | X | X | X | X | X | ||||||
| Maryland | X | X | X* | X | X | X* | X | X | ||||
| Massachusetts | X | X | X | X | X | X | X | X | ||||
| Michigan | X | X | X | X | X | X | X* | X | X | |||
| Minnesota | X | X | X | X | X | X | X | X | ||||
| Mississippi | X | X | X | X | X | X | X | X | ||||
| Missouri | X | X | X | X | X | X | X | |||||
| Montana | X | X | X | X | X | X | X | X | X | |||
| Nebraska | X | X | X | X | X | |||||||
| Nevada | X | X | X | X* | X | X | ||||||
| New Hampshire | X | X | X | X | X | X | ||||||
| New Jersey | X | X | X | |||||||||
| New Mexico | X | X | X | |||||||||
| New York | X | X | X | X | X | X | X | X | ||||
| North Carolina | X | X | X | X | X | X | X | X | X | X | ||
| North Dakota | X | X | X | X | X | X | X | X | X | X | X | |
| Ohio | X | X | X | X | X | X | X | X | X | X | ||
| Oklahoma | X | X | X | X | X | X | ||||||
| Oregon | X | X | X | X | X | X | X* | X | X | X | ||
| Pennsylvania | X | X | X | X | X | X | X | X | X | |||
| Rhode Island | X* | X | X | X | X | X | X | X* | X | X | ||
| South Carolina | X | X | X | X | X | X | X | X | ||||
| South Dakota | X | X | X | X | ||||||||
| Tennessee | X | X | X | X | X | X | X | X | X | X | ||
| Texas | X | X | X | X | X* | X | X | |||||
| Utah | X | X | X | X | ||||||||
| Vermont | X | X | X | X | X | X | X | X | ||||
| Virginia | X | X | X | X | X | X | X | X | X | |||
| Washington | X* | X | X | X | ||||||||
| West Virginia | X | X | X | X | X | X | ||||||
| Wisconsin | X | X | X | X | X | X | X | |||||
| Wyoming | X | X | X | X | X | X | ||||||
| Totals | 48 | 29 | 46 | 22 | 34 | 7 | 32 | 19 | 28 | 9 | 50 | 37 |
NOTES: States were asked to report whether they had select pharmacy benefit management strategies in place in their FFS programs in FY 2017, and/or had plans to adopt or expand these strategies in FY 2018. “*” indicates that a policy was newly adopted in FY 2018, meaning that the state did not have any policy in that category/column in place in FY 2017.SOURCE: Kaiser Family Foundation Survey of Medicaid Officials in 50 states and DC conducted by Health Management Associates, October 2017. | ||||||||||||
TABLE 19: MEDICAID FFS PHARMACY BENEFIT MANAGEMENT STRATEGIES FOR NALOXONE IN ALL 50 STATES AND DC, IN PLACE IN FY 2017 AND ACTIONS TAKEN IN FY 2018
| States | Naloxone Available ‒ At Least One Formulation Without PA | Naloxone Nasal Spray Covered Without PA | Naloxone Nasal Atomizer Covered Without PA | Naloxone Auto-Injectors Covered Without PA | Naloxone Covered for Family/Friends Obtaining Scripts on Enrollee’s Behalf | Any Naloxone Strategies | ||||||
| In place FY 2017 | New/Exp in FY 2018 | In place FY 2017 | New/Exp in FY 2018 | In place FY 2017 | New/Exp in FY 2018 | In place FY 2017 | New/Exp in FY 2018 | In place FY 2017 | New/Exp in FY 2018 | In place FY 2017 | New/Exp in FY 2018 | |
| Alabama | X | X | X | |||||||||
| Alaska | X | X | X | X | ||||||||
| Arizona | X | X | X | X | ||||||||
| Arkansas | X | X | ||||||||||
| California | X | X | X | |||||||||
| Colorado | X | X | X | X | ||||||||
| Connecticut | X | X | X | X | X | |||||||
| Delaware | X | X | ||||||||||
| DC | X* | X | ||||||||||
| Florida | X | X | X | X | X | |||||||
| Georgia | X | X | ||||||||||
| Hawaii | ||||||||||||
| Idaho | X | X | X | X | ||||||||
| Illinois | ||||||||||||
| Indiana | X | X | X | X | X | |||||||
| Iowa | X | X | X | X | ||||||||
| Kansas | X | X | X | |||||||||
| Kentucky | X | X | X | X | ||||||||
| Louisiana | X | X | X | X | X | |||||||
| Maine | ||||||||||||
| Maryland | X | X | X | X | X | |||||||
| Massachusetts | X | X | X | X | X | |||||||
| Michigan | X | X | X | X | ||||||||
| Minnesota | X | X | X | X | X | |||||||
| Mississippi | X | X | X | |||||||||
| Missouri | X | X | X | X | X | |||||||
| Montana | X | X | X | |||||||||
| Nebraska | X | X | X | |||||||||
| Nevada | X | X | X | X | X | X | ||||||
| New Hampshire | X | X | X | X | ||||||||
| New Jersey | X | X | X | X | X | X | ||||||
| New Mexico | X | X | X | X | X | X | ||||||
| New York | X | X | X | X | ||||||||
| North Carolina | X | X | X | X | X | |||||||
| North Dakota | X | X | X | |||||||||
| Ohio | X | X | X | |||||||||
| Oklahoma | X | X | X | |||||||||
| Oregon | X | X | X | X | ||||||||
| Pennsylvania | X | X | X | X | ||||||||
| Rhode Island | X | X | X | X | ||||||||
| South Carolina | X | X | X | X | ||||||||
| South Dakota | X | X | X | |||||||||
| Tennessee | ||||||||||||
| Texas | X | X | X | X | ||||||||
| Utah | X | X | X | |||||||||
| Vermont | X | X | X | X | ||||||||
| Virginia | X | X | X | |||||||||
| Washington | X | X | X | X | ||||||||
| West Virginia | X | X | X | |||||||||
| Wisconsin | X | X | X | X | ||||||||
| Wyoming | X | X | X | |||||||||
| Totals | 46 | 2 | 42 | 1 | 20 | 0 | 10 | 0 | 11 | 0 | 46 | 3 |
| NOTES: States were asked to report whether they had select pharmacy benefit management strategies in place in their FFS programs in FY 2017, and/or had plans to adopt or expand these strategies in FY 2018. “*” indicates that a policy was newly adopted in FY 2018, meaning that the state did not have any policy in that category/column in place in FY 2017. Three states (LA, MD, and MN) reported ending coverage of naloxone auto-injectors in FY 2018 and one state (NV) changed to non-preferred status.SOURCE: Kaiser Family Foundation Survey of Medicaid Officials in 50 states and DC conducted by Health Management Associates, October 2017. | ||||||||||||
Report: Administrative Challenges, Priorities, And Conclusion
Challenges and Priorities in FY 2018 and Beyond Reported by Medicaid Directors
As one Medicaid director noted, “this is an extremely difficult and challenging time for Medicaid directors.” In FY 2018, the normal challenges of administering a complex program that consumes a large share of the state’s budget have been exacerbated by the uncertainty generated by debates at the federal level regarding the future of Medicaid policy and financing. The challenge of continuing to move forward on state level initiatives under these circumstances was echoed by a number of states. States therefore reported pressing ahead on a wide variety of priorities for FY 2018 and beyond, including: pursuing new Section 1115 demonstration waivers; implementing payment and delivery system reform initiatives; enhancing access to and delivery of behavioral health services, with a specific focus on tackling the opioid epidemic; implementing long-term services and supports reforms and improvements; and executing major systems projects.
Federal Legislative Proposals
ACA Medicaid Expansion
This year’s survey was conducted as Congress debated proposals to repeal major portions of the ACA, including the ACA’s Marketplace and Medicaid coverage expansions, as well as other proposals to fundamentally restructure Medicaid’s financing structure. The survey asked states about the implications of these proposals.
Most Medicaid directors from the 32 ACA Medicaid expansion states reported that they would not be able to continue covering the expansion population, or that coverage would be at substantial risk, if the ACA enhanced federal match for this population were terminated. These coverage losses would increase the number of uninsured. Medicaid directors also pointed to budget gaps and broader state economic consequences as well as increases in uncompensated care for hospitals and FQHCs without the expansion. A number of directors also highlighted the potential negative impacts on access to behavioral health services (especially, states’ ability to address the opioid epidemic). Directors also noted the difficulty of rolling back the expanded behavioral health services and delivery system changes that have been implemented in conjunction with the Medicaid coverage expansion. Two directors (from Minnesota and New York) also noted that coverage under their Basic Health Plans would also be at risk if the ACA were repealed. A few non-expansion state Medicaid directors reported that expansion discussions were currently on hold, or had been delayed, by the ongoing federal reform debate.
Proposed Federal Medicaid Financing Reforms
Federal legislative proposals under debate at the time of the survey called for fundamental changes in Medicaid financing by converting the current open-ended matching structure to a capped financing program under a per capita cap or block grant designed to ensure federal savings.
Almost all Medicaid directors expressed concern about the likely negative fiscal consequences tied to proposed limits on federal Medicaid spending. Medicaid directors were concerned about budget shortfalls and the potential need to make program cuts and reductions (e.g., to provider rates, optional benefits, and optional eligibility pathways) as they anticipated that inflation factors included in the proposals would not be adequate to cover projected costs, particularly for new and expensive pharmaceuticals and medical treatments, growth in the aging population who have greater needs for LTSS, or public health emergencies. A number of directors expressed concern that the caps would not account for current and past state efforts, including LTSS rebalancing and movement to risk-based managed care that have helped to contain program costs but would ultimately depress a state’s base year amount used to calculate state federal funding cap amounts under a per capita cap formula. Some Medicaid directors commented that the formulas should reward efficient states. Some directors mentioned that they welcomed potential new state policy flexibility under federal legislative proposals, but a greater number of Medicaid directors expressed concern that proposals to convert Medicaid to a per capita cap or block grant would not provide sufficient flexibility to enable states to make up for the reduction in federal funds. The National Association of Medicaid Directors has issued statements on recent legislative proposals and more broadly on health reform calling for Congress to carefully consider the impacts of legislative proposals on states.126
Section 1115 Medicaid Demonstration Waivers
All new administrations can shape Medicaid through administrative actions and Section 1115 demonstration waivers. In March 2017, the Trump administration sent a letter to state governors127 that signaled a willingness to use Section 1115 authority to “support innovative approaches to increase employment and community engagement” and “align Medicaid and private insurance policies for non-disabled adults.” The letter indicates a willingness to expand these policies to traditional Medicaid adults as well as a willingness to approve landmark program changes, like work requirements.128
While previous sections of this report capture Section 1115-related policy actions planned for implementation in FY 2018, the survey also asked states whether they are planning program changes under Section 1115 authority that would be implemented after FY 2018. Nearly half of states reported activity planned for implementation after FY 2018 – as part of Section 1115 waivers currently pending at CMS, Section 1115 concept papers submitted to CMS, or more preliminary waiver ideas/concepts still under development at the state level.129 Waiver components under consideration include a range of policies such as premiums and cost-sharing (including HSA-like accounts), work requirements, healthy behavior incentives, retroactive coverage waivers, behavioral health services and systems reform, and NEMT waivers. A few examples follow:
- Kentucky131 has a waiver pending that seeks changes to its traditional Medicaid expansion. The state proposes implementing sliding scale premiums, requiring premium payment before coverage is effective, and locking those above 100 percent FPL out of coverage for six months for premium non-payment. The state seeks to require work as a condition of eligibility for most adults, proposes locking beneficiaries out of coverage for six months for failure to timely renew eligibility, and proposes waiving NEMT (an otherwise required benefit). Kentucky also submitted an amendment to its pending application. The amendment seeks to change the work requirement from a graduated requirement132 to a flat 20 hour/week requirement, adds disenrollment and lock-out provisions for failure to timely report changes to income or employment or for making false statements involving work verification, and removes a proposed expansion of presumptive eligibility sites included in the original waiver application.133
- Massachusetts has submitted an amendment to its MassHealth waiver to better align coverage with commercial plans. Under the terms of the amendment, the state would enroll higher income, non-disabled adults in the state’s Marketplace and prohibit Medicaid enrollment for certain populations with access to affordable employer coverage. The state also proposes to eliminate some of the wrap around benefit requirements for premium assistance, increase cost-sharing, implement narrow provider networks in MassHealth’s Primary Care Clinician Plan (to encourage enrollment in ACOs and MCOs instead), establish a closed formulary focused on drug efficacy, and leverage a specialty pharmacy network to reduce drug costs.134
- Wisconsin135 has submitted an amendment to its BadgerCare Reform demonstration.136 As directed by state law, they seek to amend their existing waiver for childless adults to require monthly premiums for childless adults from 51 percent to 100 percent FPL, with a coverage lock-out of up to six months for non-payment. The state proposes to offer premium reductions for completion of a health risk assessment and healthy behavior program. The state also seeks to require, as a condition of eligibility, that childless adults complete a drug screening, and if indicated, a drug test at application and renewal and would require childless adults ages 19 to 49 to work or participate in job training for 80 hours per month. In addition, the state seeks to limit childless adults’ eligibility to 48 months followed by a six-month lock-out,137 proposes to use Medicaid funds to pay for residential SUD treatment up to 90 days in institutions for mental disease for all Medicaid enrollees, and seeks authority to charge an $8 copay for emergency department utilization by childless adults.138
Concept Papers
- Alaska has developed a waiver concept paper that proposes a comprehensive behavioral health system transformation with increased access to behavioral health screening, intervention, and support services in community-based settings and via telehealth. It also proposes enhanced behavioral health services to targeted populations, such as “super-utilizers”, the homeless, and justice-involved populations, and integrating behavioral and physical health care through a new Administrative Services Organization (ASO) arrangement.139
- New Mexico reported plans to make targeted modifications to improve its existing Centennial Care managed care program. These include but are not limited to LTSS reforms to support improved care transitions, a uniform benefit package for most Medicaid adults, enhanced care coordination for justice-involved and other target populations, expanded healthy behavior incentives, a waiver of retroactive coverage requirements, and fees for missed appointments.140 The state also reported that it would like to create a DSRIP-like program for nursing homes.
Other State Priorities and Challenges
Payment and Delivery System Reform Initiatives
As noted in previous survey reports, many states are continuing to develop and implement significant initiatives that restructure delivery systems and payment structures with the goals of improving the quality of care and patient health outcomes and containing costs. One director anticipated that since federal Medicaid funding may be reduced in the future, it was more important than ever to keep making progress on delivery system reform and value-based purchasing efforts. Payment and delivery system reform efforts mentioned include value-based purchasing approaches (e.g., alternative provider payment models (APMs)), efforts to integrate physical and behavioral health, managed care expansions and reforms, integrated care partnership initiatives that engage providers at the point of service to improve care for patients, Accountable Care Organization initiatives, and multi-payer quality efforts.
Substance Use Disorder (SUD) Treatment Initiatives
With overdose deaths across the country continuing to increase, a majority involving opioids (including prescription opioids and heroin), many states are taking steps through Medicaid and other channels to reverse these trends. A number of Medicaid directors identified addressing the opioid epidemic or expanding SUD treatment efforts as a top Medicaid priority, including directors from a number of states that are seeking federal waiver authority to offer residential SUD services.
Long-Term Services and Supports
Medicaid is the nation’s primary payer for long-term services and supports (LTSS) and LTSS is also a major cost driver in state Medicaid budgets. It is therefore not surprising that a number of Medicaid directors identified LTSS reforms as a top priority for FY 2018 and beyond. Some of the initiatives mentioned included MLTSS efforts, 1915(c) waiver redesign projects, rebalancing initiatives, and other LTSS redesign efforts.
Medicaid Infrastructure Development
Most Medicaid programs have undertaken major system development projects in recent years, most notably for new eligibility systems and for new Medicaid Management Information Systems (MMIS). Several states listed the development and operationalization of these projects as a major priority in FY 2018. These Medicaid infrastructure initiatives are critically important for the success of the major delivery system and payment reforms that are often being implemented concurrently. Medicaid programs also need the systems capability to implement quality improvement, provider and MCO monitoring, data analytics, and cost control strategies.
Conclusion
This report provides information about the current landscape of state policy decisions for Medicaid during a time of great uncertainty about the future of the Medicaid program, as Congress may continue to consider reforms that could substantially roll back coverage in many states and dramatically change the financing structure of the program which has been the foundation of the federal-state Medicaid partnership. While some states are pursuing opportunities to reshape this partnership through Section 1115 demonstration waivers, others are continuing to press ahead with efforts to rebalance their long-term services and supports systems, and with delivery system and payment initiatives designed to improve health care and health outcomes and lower costs. At the same time, many states are mobilizing to address the nation’s continuing opioid epidemic by utilizing their Medicaid programs to expand access to substance use disorder treatment. Based on the findings of this survey, state Medicaid programs continue to take significant actions, both large and small, to move toward greater value, better health, and improved service for the over one-in-five Americans who are now served by the program.
Methods
The Kaiser Family Foundation (KFF) commissioned Health Management Associates (HMA) to survey Medicaid directors in all 50 states and the District of Columbia to identify and track trends in Medicaid spending, enrollment, and policy making. This is the 17th annual survey, each conducted at the beginning of the state fiscal year from FY 2002 through FY 2017. Additionally, eight mid-fiscal year surveys were conducted during state fiscal years 2002-2004 and 2009-2013, when a large share of states were considering mid-year Medicaid policy changes due to state budget and revenue shortfalls. Findings from previous surveys are referenced in this report when they help to highlight current trends. Archived copies of past reports are available on the following page.141
The KFF/HMA Medicaid survey on which this report is based was conducted from June through September 2017. The survey instrument (in the Appendix) was designed to document policy actions in place in FY 2017 and implemented or adopted for FY 2018 (which began for most states on July 1, 2017).142 The survey captures information consistent with previous surveys, particularly for eligibility, provider payment rates, benefits, long-term care, and managed care to provide some trend information. Each year, questions are added to address current issues.
Medicaid directors and staff provided data for this report in response to a written survey and a follow-up telephone interview. The survey was sent to each Medicaid director in June 2017. All 50 states and DC completed surveys and participated in telephone interview discussions in July, August, and September 2017. The telephone discussions are an integral part of the survey to ensure complete and accurate responses and to record the complexities of state actions.
The survey does not attempt to catalog all Medicaid policies in place for each state. The focus is on changes in Medicaid policy and new initiatives that are planned for FY 2018. Experience has shown that adopted policies are sometimes delayed or not implemented, for reasons related to legal, fiscal, administrative, systems or political considerations, or due to delays in approval from CMS. Policy changes under consideration without a definite decision to implement are not included in the survey. The District of Columbia is counted as a state for the purposes of this report; the counts of state policies or policy actions that are interspersed throughout this report include survey responses from the 51 “states” (including DC). Given differences in the financing structure of their programs, the U.S. territories were not included in this analysis.
Glossary
Acronym Glossary
AAC – Actual Acquisition Cost
ACA – Affordable Care Act
ACO – accountable care organization
ASO – Administrative Services Organization
APCD – all-payer claims database
APM – alternative payment model
BH – behavioral health
CDC – The Centers for Disease Control and Prevention
CFC – Community First Choice
CHIP – Children’s Health Insurance Program
CHIPRA – Children’s Health Insurance Program Reauthorization Act of 2009
CMS – The Centers for Medicare and Medicaid Services
CON – Certificate of Need
CSHCNs – children with special health care needs
DBM – dental benefit manager
D-SNP – Medicare Dual Eligible Special Needs Plans
DSRIP – Delivery System Reform Incentive Program
DUR – drug utilization review
EAC – Estimated Acquisition Cost
ED – emergency department
EPSDT – Early and Periodic Screening, Diagnostic, and Treatment
FAD – Financial Alignment Demonstration
FDA – Food and Drug Administration
FFS – fee-for-service
FFY – federal fiscal year
FIDE-SNP – Fully Integrated Dual Eligible Special Needs Plans
FPL – federal poverty level
FQHC – federally qualified health center
FY – state fiscal year
GED – general educational development or diploma
HSA – health savings account
HCBS – home and community-based services
HEDIS – Healthcare Effectiveness Data and Information Set
HIT – health information technology
ICF-ID – intermediate care facilities for individuals with intellectual disabilities
ID/DD – intellectual and developmental disabilities
IEP – individualized education program
IMD – institutions for mental diseases
LTSS – long-term services and supports
MAGI – modified adjusted gross income
MAT – medication-assisted treatment
MCO – managed care organization
MED – morphine equivalent dose
MFP – Money Follows the Person (federal grant program)
MH – mental health
MLTSS – managed long-term services and supports
MLR – medical loss ratio
MME – morphine milligram equivalent
MMIS – Medicaid Management Information System
NADAC – National Average Drug Acquisition Costs
NCQA – National Committee for Quality Assurance
NEMT – non-emergency medical transportation
NF – nursing facility
OT – occupational therapy
P4P – pay for performance
PA – prior authorization
PACE – Programs of All-Inclusive Care for the Elderly
PCCM – primary care case management
PCMH – patient-centered medical home
PDL – preferred drug list
PDMP – Prescription Drug Monitoring Program
PHP – prepaid health plan
PIP – performance improvement projects
PMPM – per-member per-month
PT – physical therapy
RHC – rural health center
SED – serious emotional disturbance
SIM – State Innovation Models federal grant program
SMI – serious mental illness
SNAP – Supplemental Nutrition Assistance Program
SPA – State Plan Amendment
SSI – supplemental security income
SUD – substance use disorder
TPL – third party liability
VBP – value-based purchasing
WIC – Special Supplemental Nutrition Program for Women, Infants, and Children
Appendix
Survey Instrument
Download the Survey (.pdf)
Endnotes
- Medicaid work requirement proposals generally require beneficiaries to verify their participation in approved activities, such as employment, job search, or job training programs, for a certain number of hours per week to receive health coverage. The proposals typically would exempt certain populations. To date, CMS has not approved state waiver requests to require that Medicaid beneficiaries work as a condition of eligibility. ↩︎
- While still maintaining the enhanced federal matching rate for coverage of the remaining expansion population at or below 100% FPL. ↩︎
- Six states (CA, MA, MD, VA, VT, and WV) currently have received federal approval through Section 1115 waiver authority to waive the IMD payment exclusion to receive federal Medicaid funds for inpatient behavioral health services for nonelderly adults. Nine states (AZ, IL, IN, KY, MA, MI, NJ, WI, and UT) currently have pending Section 1115 waivers at CMS which seek to waive the IMD payment exclusion. ↩︎
- AR and IL did not respond to the MAT drug coverage question; however, a Health Affairs article (citation below) that uses 2013-2014 data indicates that all 51 states cover buprenorphine. Colleen Grogan et al., “Survey Highlights Differences in Medicaid Coverage for Substance Use Treatment and Opioid Use Disorder Medications,” Health Affairs 35 no. 12 (December 2016): 2289-2296, http://content.healthaffairs.org/content/35/12/2289. ↩︎
- Centers for Medicare & Medicaid Services. National Health Expenditures (Washington, DC: Centers for Medicare & Medicaid Services, December 2016), https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical.html. ↩︎
- State fiscal years begin on July 1 except for these states: NY on April 1; TX on September 1; AL, MI and DC on October 1. ↩︎
- Kaiser Family Foundation, 50-State Medicaid Budget Survey Archives, (Washington, DC: Kaiser Family Foundation, October 2017), https://modern.kff.org/medicaid/report/medicaid-budget-survey-archives/. ↩︎
- While still maintaining the enhanced federal matching rate for coverage of the remaining expansion population at or below 100% FPL. ↩︎
- Legislators dropped their lawsuit challenging Governor Cooper’s authority to expand Medicaid without legislative approval in July 2017 because Cooper never formally submitted his expansion proposal to the federal government for review. However, in a joint statement, the State Senate President and Speaker of the State House said that they would renew a legal challenge if the Governor made another attempt to expand without lawmakers’ approval. ↩︎
- Maine Question 2, Medicaid Expansion Initiative, (Ballotpedia, 2017), https://ballotpedia.org/Maine_Question_2,_Medicaid_Expansion_Initiative_(2017). ↩︎
- Individuals eligible for the Family Planning Waiver included women ages 18 through 55 who have family income at or below 185 percent of the federal poverty level (FPL) and assets totaling less than $250,000, and who are not otherwise eligible for Medicare, Medicaid, the Children’s Health Insurance Program (CHIP), or health insurance coverage that provides family planning services. ↩︎
- Missouri has replaced its family planning waiver with a state-funded family planning coverage program that does not cover or pay for services provided by organizations that also provide abortion services, including Planned Parenthood. Women who are eligible for the federally-funded program will continue to be eligible for the state-funded program, without change. The available services remain the same but the provider qualifications are modified. Missouri Department of Social Services, Public Notice of Suspension of Federal Expenditure Authority for Section 1115 Family Planning Demonstration, entitled “Missouri Woman’s Health Services Program,” (Missouri Department of Social Services, July 2016), https://dss.mo.gov/mhd/waivers/1115-demonstration-waivers/files/missouri-women-health-services-waiver-suspension-notice-phase-out-plan.pdf. ↩︎
- Thomas Price and Seema Verma letter to governors, March 14, 2017, https://www.hhs.gov/sites/default/files/sec-price-admin-verma-ltr.pdf. ↩︎
- Elizabeth Hinton, MaryBeth Musumeci, Robin Rudowitz, and Larisa Antonisse, Section 1115 Medicaid Demonstration Waivers: A look at the Current Landscape of Approved and Pending Waivers, (Washington, DC: Kaiser Family Foundation, September 2017), https://modern.kff.org/medicaid/issue-brief/section-1115-medicaid-demonstration-waivers-a-look-at-the-current-landscape-of-approved-and-pending-waivers/. ↩︎
- States with pending waiver proposals with provisions slated for implementation after FY 2018 include Alaska, Colorado, Illinois, Indiana, Kentucky, Maine, Massachusetts, New Mexico, North Carolina, Oklahoma, Virginia, and Wisconsin. ↩︎
- MaryBeth Musumeci, Elizabeth Hinton, and Robin Rudowitz, Section 1115 Medicaid Expansion Waivers: A Look at Key Themes and State Specific Waiver Provisions (Washington, DC: Kaiser Family Foundation, August 2017), https://modern.kff.org/medicaid/issue-brief/section-1115-medicaid-expansion-waivers-a-look-at-key-themes-and-state-specific-waiver-provisions/. ↩︎
- MaryBeth Musumeci, Elizabeth Hinton, and Robin Rudowitz, Section 1115 Medicaid Expansion Waivers: A Look at Key Themes and State Specific Waiver Provisions (Washington, DC: Kaiser Family Foundation, August 2017), https://modern.kff.org/medicaid/issue-brief/section-1115-medicaid-expansion-waivers-a-look-at-key-themes-and-state-specific-waiver-provisions/. ↩︎
- CMS’s waiver of retroactive eligibility in Arkansas is conditioned on the state completing an eligibility determination mitigation plan, making timely eligibility determinations, providing benefits during a reasonable opportunity period for otherwise eligible individuals who attest to immigration status, and implementing a hospital presumptive eligibility program. ↩︎
- MaryBeth Musumeci, Elizabeth Hinton, and Robin Rudowitz, Section 1115 Medicaid Expansion Waivers: A Look at Key Themes and State Specific Waiver Provisions (Washington, DC: Kaiser Family Foundation, August 2017), https://modern.kff.org/medicaid/issue-brief/section-1115-medicaid-expansion-waivers-a-look-at-key-themes-and-state-specific-waiver-provisions/. ↩︎
- The member can reenroll within 90 days from the end of the expired benefit period if they submit the requested redetermination information. However, after the 90-day period, the member is required to wait another three months, or six months from the initial date of disenrollment, until their next open enrollment before being permitted to reenroll in HIP. Indiana has also proposed a work requirement, but that provision would not be effective until FY 2019. ↩︎
- As of the date of publication, the state’s waiver amendment was still pending approval at CMS. ↩︎
- MaryBeth Musumeci, Elizabeth Hinton, and Robin Rudowitz, Proposed Medicaid Section 1115 Waivers in Maine and Wisconsin (Washington, DC: Kaiser Family Foundation, updated August 2017), https://modern.kff.org/medicaid/issue-brief/proposed-medicaid-section-1115-waivers-in-maine-and-wisconsin/. ↩︎
- The full waiver application is available at https://www.medicaid.gov/Medicaid-CHIP-Program-Information/By-Topics/Waivers/1115/downloads/me/me-mainecare-pa.pdf , and a summary of its provisions is available at http://modern.kff.org/medicaid/issue-brief/proposed-medicaid-section-1115-waivers-in-maine-and-wisconsin/. ↩︎
- Enrollment in the PCN limited benefit package for adults up to 100% FPL is capped at 25,000. ↩︎
- Although these policies are similar to policies in other states that are counted as eligibility restrictions in this report, they are not counted as eligibility restrictions in Utah because they apply only to the new childless adult group that does not currently have access to coverage. ↩︎
- Maine’s pending Section 1115 waiver proposes requiring monthly premiums for traditional adults (such as parents, former foster care youth, those receiving TMA, medically needy, those receiving family planning services). While the waiver proposal’s estimated implementation date for most other (non-premium) provisions is January 1, 2018, the state doesn’t plan to implement the premium provisions until six months later (estimated at July 1, 2018). Given this FY 2019 anticipated implementation date, Maine’s premium proposal is not counted in this section of the report (which captures premium changes in FY 2017 and planned for FY 2018). ↩︎
- Jennifer Ryan, Lucy Pagel, Katy Smali, Samantha Artiga, Robin Rudowitz, and Alexandra Gates, Connecting the Justice-Involved Population to Medicaid Coverage and Care: Findings from Three States (Washington, DC, Kaiser Commission on Medicaid and the Uninsured, June 2016), https://modern.kff.org/medicaid/issue-brief/connecting-the-justice-involved-population-to-medicaid-coverage-and-care-findings-from-three-states/. ↩︎
- Some states suspend by limiting covered benefits to inpatient hospitalization. ↩︎
- Positive changes from the beneficiary’s perspective that were counted in this report are denoted with (+). Negative changes from the beneficiary’s perspective that were counted in this report are denoted with (-). Reductions to Medicaid eligibility pathways in response to the availability of other coverage options (including Marketplace or Medicaid expansion coverage) were denoted as (#). ↩︎
- New premiums are denoted as (New). Changes to premium policies that have a neutral impact from the beneficiary’s perspective are denoted as (Neutral). ↩︎
- This table captures eligibility and premium changes that states have implemented or plan to implement in FY 2017 or 2018, including changes that are part of pending Section 1115 waivers. For pending waivers, only provisions planned for implementation before the end of FY 2018 (according to waiver application documents) are counted in this table. Waiver provisions in pending waivers that states plan to implement in FY 2019 or after are not counted here. ↩︎
- CMS’s waiver of retroactive eligibility in Arkansas is conditioned on the state completing an eligibility determination mitigation plan, making timely eligibility determinations, providing benefits during a reasonable opportunity period for otherwise eligible individuals who attest to immigration status, and implementing a hospital presumptive eligibility program. ↩︎
- Massachusetts’ pending amendment would remove an existing waiver provision that allows it to enroll expansion adults and other populations in coverage during a 90-day provisional eligibility period while income verification is pending. ↩︎
- Six states (CA, MA, MD, VA, VT, and WV) currently have received federal approval through Section 1115 waiver authority to waive the IMD payment exclusion to receive federal Medicaid funds for inpatient behavioral health services for nonelderly adults. Nine states (AZ, IL, IN, KY, MA, MI, NJ, WI, and UT) currently have pending Section 1115 waivers at CMS which seek to waive the IMD payment exclusion. ↩︎
- Julia Paradise and MaryBeth Musumeci, CMS’s Final Rule on Medicaid Managed Care: A Summary of Major Provisions (Washington, DC: Kaiser Family Foundation, June 2016), http://files.kff.org/attachment/CMSs-Final-Rule-on-Medicaid-Managed-Care. ↩︎
- The general effective date of the final rule is July 5, 2016, although individual provisions of the rule take effect at different times. ↩︎
- Brian Neale, Medicaid Managed Care Regulations with July 1, 2017 Compliance Dates, (Center for Medicaid and CHIP Services Informational Bulletin, June 2017), https://www.medicaid.gov/federal-policy-guidance/downloads/cib063017.pdf. ↩︎
- Connecticut does not have capitated managed care arrangements, but does carry out many managed care functions, including ASO arrangements, payment incentives based on performance, intensive care management, community workers, educators, and linkages with primary care practices. ↩︎
- California has a small PCCM program operating in LA County for those with HIV. Three states use PCCM authority to operate specialized programs that are not counted here as PCCM programs: South Carolina uses PCCM authority to provide care management services to approximately 200 medically complex children; the Texas Medicaid Wellness program provides care management services for high-cost/high-risk enrollees, and Wyoming’s Patient Centered Medical Home program uses PCCM authority to make PMPM payments. ↩︎
- Centers for Medicare and Medicaid Services, Medicaid & CHIP Monthly Application, Eligibility Determinations, and Enrollment Reports, (Washington, DC: Centers for Medicare and Medicaid Services, May 2017), http://www.medicaid.gov/medicaid-chip-program-information/program-information/medicaid-and-chip-enrollment-data/medicaid-and-chip-application-eligibility-determination-and-enrollment-data.html. ↩︎
- Arizona was re-characterized from “Varies” to “Always Mandatory” across all population groups as the only non-mandatory group is Native Americans in compliance with federal requirements. ↩︎
- 81 FR 27497, available at: https://www.gpo.gov/fdsys/granule/FR-2016-05-06/2016-09581. ↩︎
- In the rule, CMS formalizes its policy around “in lieu of,” which is an authority that a number of states were using to cover stays in IMDs prior to this rule. Some of these states must now adapt policies to meet the 15-day requirement, which may have fiscal and programmatic implications for these states. ↩︎
- Six states (CA, MA, MD, VA, VT, and WV) currently have received federal approval through Section 1115 waiver authority to waive the IMD payment exclusion to receive federal Medicaid funds for inpatient behavioral health services for nonelderly adults. Nine states (AZ, IL, IN, KY, MA, MI, NJ, WI, and UT) currently have pending Section 1115 waivers at CMS which seek to waive the IMD payment exclusion. ↩︎
- National Association of Medicaid Directors, Medicaid Value-Based Purchasing: What Is It & Why Does It Matter? (Washington, DC: National Association of Medicaid Directors, January 2017), http://medicaiddirectors.org/wp-content/uploads/2017/01/Snapshot-2-VBP-101_FINAL.pdf. ↩︎
- For more information on the State Innovation Models (SIM) initiative, see: https://innovation.cms.gov/initiatives/state-innovations/. ↩︎
- Public Hospital Redesign and Incentives in Medi-Cal (PRIME) program: http://www.dhcs.ca.gov/provgovpart/Pages/PRIME.aspx. ↩︎
- Centers for Medicare and Medicaid Services, CMS’ Accountable Health Communities Model selects 32 participants to serve as local “hubs”, (Baltimore, MD: Centers for Medicare and Medicaid Services, April 2017), https://www.cms.gov/Newsroom/MediaReleaseDatabase/Press-releases/2017-Press-releases-items/2017-04-06.html. ↩︎
- Centers for Medicare and Medicaid Services, Medicaid and CHIP Managed Care Final Rule (CMS 2390-F) Implementation Dates (Baltimore, MD: Centers for Medicare and Medicaid Services, April 2017), https://www.medicaid.gov/medicaid/managed-care/downloads/implementation-dates.pdf. ↩︎
- Remittances are required for the expansion population in Ohio. ↩︎
- Texas reported ending its Texas Medicaid Wellness Program in August 2017. This care management program was not counted as a PCCM program for purposes of this report although it operated under PCCM authority. ↩︎
- One of the 25 states reporting a PHP arrangement that is not included in Exhibit 5 is Alabama, which reported having a PHP for maternity care. ↩︎
- “Patient-Centered Medical Home Recognition,” National Committee on Quality Assurance, accessed October 1, 2015, http://www.ncqa.org/Programs/Recognition/Practices/PatientCenteredMedicalHomePCMH.aspx. ↩︎
- Kaiser Commission on Medicaid and the Uninsured, Medicaid Delivery System and Payment Reform: A Guide to Key Terms and Concept (Washington, DC: Kaiser Commission on Medicaid and the Uninsured, June 2015), https://modern.kff.org/medicaid/fact-sheet/medicaid-delivery-system-and-payment-reform-a-guide-to-key-terms-and-concepts/. ↩︎
- In this report, Oregon’s Coordinated Care Organization (CCO) program is counted as an MCO program, but not as an ACO program, consistent with its CMS designation and the state’s survey response. According to the state, “A coordinated care organization is a network of all types of health care providers (physical health care, addictions and mental health care and sometimes dental care providers) who have agreed to work together in their local communities to serve people who receive health care coverage under the Oregon Health Plan (Medicaid).” (Oregon Health Authority website accessed at: http://www.oregon.gov/oha/HPA/Pages/CCOs-Oregon.aspx.) ↩︎
- Kaiser Commission on Medicaid and the Uninsured, Medicaid Delivery System and Payment Reform: A Guide to Key Terms and Concept (Washington, DC: Kaiser Commission on Medicaid and the Uninsured, June 2015), https://modern.kff.org/medicaid/fact-sheet/medicaid-delivery-system-and-payment-reform-a-guide-to-key-terms-and-concepts/. ↩︎
- Samantha Artiga, Robin Rudowitz, Jennifer Tolbert, Julia Paradise, and Melissa Majerol, Findings from the Field: Medicaid Delivery Systems and Access to Care in Four States in Year Three of the ACA (Washington, DC, Kaiser Commission on Medicaid and the Uninsured, September 2016), https://modern.kff.org/report-section/findings-from-the-field-medicaid-delivery-systems-and-access-to-care-in-four-states-in-year-three-of-the-aca-issue-brief/. ↩︎
- Rhode Island Executive Office of Health and Human Services (EOHHS), Medicaid Program Accountable Entity Roadmap Document (EOHHS, April 2017), accessed at: http://www.eohhs.ri.gov/Portals/0/Uploads/Documents/Acc_Entitites/MedicaidAERoadmap.pdf. ↩︎
- Alexandra Gates, Robin Rudowitz, and Jocelyn Guyer, An Overview of Delivery System Reform Incentive Payment (DSRIP) Waivers (Washington, DC, Kaiser Commission on Medicaid and the Uninsured, September 2014), https://modern.kff.org/report-section/findings-from-the-field-medicaid-delivery-systems-and-access-to-care-in-four-states-in-year-three-of-the-aca-issue-brief/. ↩︎
- Gobeille v. Liberty Mutual Insurance Company, No. 14-181, 577 U.S. ___ (2016). In this case, the Supreme Court of the United States held that the Employee Retirement Income Security Act (ERISA) pre-empts a Vermont law that requires certain entities, including health insurers, to report payments relating to health care claims and other information relating to health care services to a state agency for compilation in an all-inclusive health care database. ↩︎
- Elizabeth Hinton and Julia Paradise, Access to Dental Care in Medicaid: Spotlight on Nonelderly Adults, (Washington, DC: Kaiser Commission on Medicaid and the Uninsured, May 2016), http://modern.kff.org/medicaid/issue-brief/access-to-dental-care-in-medicaid-spotlight-on-nonelderly-adults/. ↩︎
- Medicare Payment Advisory Commission, “Telehealth Services and the Medicare Program,” chap. 8 in Report to the Congress: Medicare and the Health Care Delivery System, (Washington, DC: June 2016), 229-260, http://www.medpac.gov/docs/default-source/reports/chapter-8-telehealth-services-and-the-medicare-program-june-2016-report-.pdf?sfvrsn=0. ↩︎
- Vikki Wachino, Coverage of Housing-Related Activities and Services for Individuals with Disabilities (Baltimore, MD: Center for Medicaid and CHIP Services Informational Bulletin, June 2015), https://www.medicaid.gov/federal-policy-guidance/downloads/CIB-06-26-2015.pdf. ↩︎
- Steve Eiken, Kate Sredl, Brian Burwell, and Rebecca Woodward, Medicaid Expenditures for Long-Term Services and Supports (LTSS) in FY 2015 (Baltimore, MD: CMS, April 2017), https://www.medicaid.gov/medicaid/ltss/downloads/reports-and-evaluations/ltssexpendituresffy2015final.pdf. ↩︎
- Steve Eiken, Kate Sredl, Brian Burwell, and Paul Saucier, Medicaid Expenditures for Long-Term Services and Supports (LTSS) in FY 2014: Managed LTSS Reached 15 Percent of LTSS Spending (Baltimore, MD: CMS, April 15, 2016), https://www.medicaid.gov/medicaid/ltss/downloads/ltss-expenditures-2014.pdf. ↩︎
- Serving more individuals through “HCBS Waivers or SPAs” means: adopting new waiver; adding and filling more waiver slots; filling more waiver slots; adding new 1915(i) or 1915(k) SPA; or serving more individuals through existing 1915(i) or 1915(k) SPA. ↩︎
- While various Medicaid state plan authorities enable states to expand beneficiary access to home and community-based services (HCBS), some states are using Section 1115 waivers to streamline program administration, improve care coordination, and expand beneficiary access to home and community-based services (HCBS). ↩︎
- Of 23 states with MLTSS. ↩︎
- “Serving more people through PACE” means: adding new provider sites and/or increasing the number of people served at existing sites. ↩︎
- CMCS Informational Bulletin, Coverage of Housing-Related Activities and Services for Individuals with Disabilities (Baltimore, MD: Center for Medicaid and CHIP Services, June 2015), https://www.medicaid.gov/federal-policy-guidance/downloads/CIB-06-26-2015.pdf. ↩︎
- After September 2016, with CMS approval, states can continue to transition eligible individuals through 2018 and expend remaining MFP funds through federal FY 2020. ↩︎
- Oregon is not included in this count. The state terminated its MFP program, effective June 30, 2015. ↩︎
- CMS provides information on Money Follows the Person at https://www.medicaid.gov/medicaid/ltss/money-follows-the-person/index.html. ↩︎
- Molly O’Malley Watts, Erica Reaves, and MaryBeth Musumeci, Money Follows the Person: A 2015 State Survey of Transitions, Services, and Costs, (Washington, DC: Kaiser Family Foundation, October 2015), http://modern.kff.org/medicaid/report/money-follows-the-person-a-2015-state-survey-of-transitions-services-and-costs/. ↩︎
- Most of these states are using current Section 1915(c) waivers that provide community transition services and environmental modifications for seniors, individuals with physical disabilities and/or individuals with intellectual or developmental disabilities, and some states offer housing coordinators or other search services to assist waiver beneficiaries. ↩︎
- “Money Follows the Person (MFP),” Centers for Medicare and Medicaid Services, accessed October 1, 2017, https://www.medicaid.gov/medicaid/ltss/money-follows-the-person/index.html. ↩︎
- Molly O’Malley Watts, MaryBeth Musumeci, and Petry Ubri, Medicaid Section 1115 Managed Long-Term Services and Supports Waivers: A Survey of Enrollment, Spending an Program Policies, (Washington, DC: Kaiser Family Foundation, January 2017), http://modern.kff.org/medicaid/report/medicaid-section-1115-managed-long-term-services-and-supports-waivers-a-survey-of-enrollment-spending-and-program-policies/. ↩︎
- U.S. Senate Commission on Long-Term Care, Report to the Congress, (U.S. Senate Commission on Long-Term Care, September 2013), https://www.gpo.gov/fdsys/pkg/GPO-LTCCOMMISSION/pdf/GPO-LTCCOMMISSION.pdf. ↩︎
- Arkansas, Delaware, and Minnesota noted they are/will be forming work/advisory groups to study the issue further. New York also reported an expanded scope of practice for home care aides. North Carolina reported consumer-directed care is included as an option in the newly approved children’s waiver. North Dakota reported ongoing efforts to address LTSS direct care workforce through its MFP program. Texas reported working with external and internal stakeholders to develop measures and methods to monitor access to MLTSS as it relates to managed care organization network adequacy. ↩︎
- HCBS benefit expansions reported in this section may include new HCBS waiver or SPA initiatives, which may have also been reported/counted as expansions in persons served under HCBS through waivers or SPAs. ↩︎
- This count does not include two states (Colorado and Washington) that have managed FFS FADs. For more information see: https://www.cms.gov/Medicare-Medicaid-Coordination/Medicare-and-Medicaid-Coordination/Medicare-Medicaid-Coordination-Office/FinancialAlignmentInitiative/ManagedFeeforServiceModel.html. ↩︎
- Rhode Island and South Carolina launched the FAD in FY 2017. ↩︎
- The Affordable Care Act (ACA) authorized the Secretary of Health and Human Services to implement the Financial Alignment Initiative to allow state-administered demonstration projects to improve the integration and coordination of services for individuals who are covered under both Medicare and Medicaid. This population, as a group, experiences high rates of hospitalization and use of LTSS and is, on average, a high need, high cost population. See: https://www.cms.gov/Medicare-Medicaid-Coordination/Medicare-and-Medicaid-Coordination/Medicare-Medicaid-Coordination-Office/FinancialAlignmentInitiative/FinancialModelstoSupportStatesEffortsinCareCoordination.html.. ↩︎
- Kaiser Commission on Medicaid and the Uninsured, Health Plan Enrollment in the Capitated Financial Alignment Demonstrations for Dual Eligible Beneficiaries (Washington, DC: Kaiser Commission on Medicaid and the Uninsured, August 2016), https://modern.kff.org/medicaid/fact-sheet/health-plan-enrollment-in-the-capitated-financial-alignment-demonstrations-for-dual-eligible-beneficiaries/. ↩︎
- Arizona, Hawaii, Massachusetts, New Mexico, Pennsylvania, Tennessee, Texas, and Virginia. (Virginia has FAD model that the state plans to terminate by the end of 2017. Virginia is also launching MLTSS program in August 2017.) ↩︎
- Dual Eligible Special Needs Plans (D-SNPs) enroll beneficiaries who are entitled to both Medicare and Medicaid and offer the opportunity to better coordinate benefits among Medicare and Medicaid. For more information see: https://www.cms.gov/Medicare/Health-Plans/SpecialNeedsPlans/DualEligibleSNP.html. ↩︎
- Fully Integrated Dual Eligible SNPs were created by Congress in Section 3205 of the Affordable Care Act to promote full integration and coordination of Medicaid and Medicare benefits for dual eligible beneficiaries by a single managed care organization. They must have a MIPPA compliant contract with a State Medicaid Agency that includes coverage of specified primary, acute and long-term care benefits and services under risk-based financing. For more information see: https://www.cms.gov/Medicare/Health-Plans/SpecialNeedsPlans/DualEligibleSNP.html#s3. ↩︎
- Delaware, Iowa, Idaho, Minnesota, and New Jersey. ↩︎
- Rates for calendar year 2017 not yet determined at the time of the survey included MCO rates for Florida, Illinois, Maryland, and Minnesota. While some states with calendar year contracts provided the budgeted level of MCO rate increases, these four states indicate that they are waiting for work by their actuaries. Wisconsin is implementing APR-DRGs in in January 2017, which could potentially move funds between inpatient and outpatient hospital rates. ↩︎
- Historically, Medicaid reimbursement for hospitals and nursing homes was cost-based, automatically reflecting incurred cost increases. When rates for these providers are frozen, such annual increases do not occur; hence for this report, rate freezes are counted as restrictions. ↩︎
- Some states also have premium or claims taxes that apply to managed care organizations and other insurers. Since this type of tax is not considered a provider tax by CMS, these taxes are not counted as provider taxes in this report. ↩︎
- In addition to the “Medicaid provider taxes” included in this report, several states have more general health care taxes that are used to fund their Medicaid programs. For instance, some states have taxes on insurance premiums or health care claims that apply to all payers. States were asked whether they have a tax on MCOs, health insurance premiums, or health care claims that does not apply to other goods and services. Thirteen states that had not indicated a Medicaid MCO provider tax replied “yes”. Two of these states indicated that all of the health insurance tax is dedicated to Medicaid. Four indicated that it was dedicated to Medicaid in part. ↩︎
- Kaiser Family Foundation, Compare Proposals to Replace the Affordable Care Act, (Washington, DC: Kaiser Family Foundation, September 2017), https://modern.kff.org/interactive/proposals-to-replace-the-affordable-care-act/. ↩︎
- Robin Rudowitz, Larisa Antonisse, and MaryBeth Musumeci, Medicaid Changes in Better Care Reconciliation Act (BCRA) Go Beyond ACA Repeal and Replace, (Washington, DC: Kaiser Family Foundation, July 2017), https://modern.kff.org/medicaid/issue-brief/medicaid-changes-in-better-care-reconciliation-act-bcra-go-beyond-aca-repeal-and-replace/. ↩︎
- Kaiser Family Foundation, Summary of Graham-Cassidy-Heller-Johnson Amendment, (Washington, DC: Kaiser Family Foundation, September 2017), http://files.kff.org/attachment/Summary-of-Graham-Cassidy-Heller-Johnson-Amendment. ↩︎
- AR and IL did not respond to the MAT drug coverage question; however, a Health Affairs article (citation below) that uses 2013-2014 data indicates that all 51 states cover buprenorphine. Colleen Grogan et al., “Survey Highlights Differences in Medicaid Coverage for Substance Use Treatment and Opioid Use Disorder Medications,” Health Affairs 35 no. 12 (December 2016): 2289-2296, http://content.healthaffairs.org/content/35/12/2289. ↩︎
- Centers for Medicare and Medicaid Services, New Service Delivery Opportunities for Individuals with a Substance Use Disorder (Baltimore, MD: CMS, July 2015), https://www.medicaid.gov/federal-policy-guidance/downloads/SMD15003.pdf. ↩︎
- BRCA1 and BRCA2 are human genes that produce tumor suppressor proteins that help repair damaged DNA and play a role in ensuring the stability of the cell’s genetic material. When either of these genes are mutated or altered, cells are more likely to develop additional genetic alterations that can lead to cancer. Specific inherited mutations in BRCA1 and BRCA2 increase the risk of female breast and ovarian cancers. More information is available from the National Cancer Institute: https://www.cancer.gov/about-cancer/causes-prevention/genetics/brca-fact-sheet. ↩︎
- Utah proposes covering a new eligibility group: individuals with income below 5 percent of the FPL who are either chronically homeless, justice-involved, or individuals in need of substance use and/or mental health treatment. This EPSDT restriction would apply to 19 and 20 year olds in this group. ↩︎
- Julia Paradise, Medicaid Moving Forward (Washington, DC: Kaiser Commission on Medicaid and the Uninsured, March 2015), https://modern.kff.org/health-reform/issue-brief/medicaid-moving-forward/. ↩︎
- MaryBeth Musumeci, Elizabeth Hinton, and Robin Rudowitz, Proposed Medicaid Section 1115 Waivers in Maine and Wisconsin (Washington, DC: Kaiser Family Foundation, updated August 2017), https://modern.kff.org/medicaid/issue-brief/proposed-medicaid-section-1115-waivers-in-maine-and-wisconsin/. ↩︎
- Katherine Young, Robin Rudowitz, Rachel Garfield, and MaryBeth Musumeci, Medicaid’s Most Costly Outpatient Drugs (Washington, DC: Kaiser Family Foundation, July 2016), https://modern.kff.org/medicaid/issue-brief/medicaids-most-costly-outpatient-drugs/. ↩︎
- In accordance with federal and state law, states pay the lower of (a) the ingredient cost rate plus a dispensing fee; (b) the Federal Upper Limit (FUL) or State Maximum Allowable Cost rate, if applicable, plus a dispensing fee; or (c) the pharmacy’s Usual and Customary Charge. ↩︎
- 81 Fed. Reg. 5170. ↩︎
- Centers for Medicare and Medicaid Services, CMCS Informational Bulletin: Medicaid Pharmacy – Survey of Retail Prices (Washington, DC: Centers for Medicare and Medicaid Services, May 2012), http://www.medicaid.gov/Federal-Policy-Guidance/Downloads/CIB-05-31-12.pdf. ↩︎
- One of the states that projected greater costs (South Carolina) reported, however, that it is negotiating with CMS to retain its current reimbursement methodology and not move to AAC and a professional dispensing fee. The state’s interpretation of the rule is that it sets an aggregate cost cap based on the AAC methodology but does not require adoption of this methodology. South Carolina believes it is compliant with the rule as its pharmacy expenditures are within the aggregate cap based on AAC, but if the state were to adopt the AAC methodology, it would incur greater costs. ↩︎
- Wisconsin Department of Health Services, Family Care, Family Care Partnership, and PACE Enrollment Data, (Wisconsin Department of Health Services, August 2017), https://www.dhs.wisconsin.gov/familycare/reports/enrollmentdata.pdf. ↩︎
- A “kick payment” is a supplemental payment over and above the capitation payment made to an MCO for beneficiaries utilizing a specified set of services or having a certain condition. ↩︎
- “Understanding the Epidemic,” Centers for Disease Control and Prevention, accessed on October 1, 2017, https://www.cdc.gov/drugoverdose/epidemic/index.html. ↩︎
- Li Hui Chen, Holly Hedegaard, and Margaret Warner, “Drug-poisoning deaths involving opioid analgesics: United States, 1999–2011,” National Center for Health Statistics data brief, no 166 (September 2014), https://www.cdc.gov/nchs/data/databriefs/db166.pdf. ↩︎
- Katherine Young and Julia Zur, Medicaid and the Opioid Epidemic: Enrollment, Spending, and the Implications of Proposed Policy Changes (Washington, DC: The Kaiser Family Foundation, July 2017), http://modern.kff.org/medicaid/issue-brief/medicaid-and-the-opioid-epidemic-enrollment-spending-and-the-implications-of-proposed-policy-changes/. ↩︎
- Center for Medicaid and CHIP Services, Best Practices for Addressing Prescription Opioid Overdoses, Misuse, and Addiction, (Baltimore, MD: CMCS Informational Bulletin, January 2016), https://www.medicaid.gov/federal-policy-guidance/downloads/cib-02-02-16.pdf. ↩︎
- Deborah Dowell, Tamara Haegerich, and Roger Chou, “CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016,” Centers for Disease Control and Prevention Morbidity and Mortality Weekly Report, 65, no. 1 (March 2016):1–49, http://dx.doi.org/10.15585/mmwr.rr6501e1. ↩︎
- Center for Medicaid and CHIP Services, Best Practices for Addressing Prescription Opioid Overdoses, Misuse, and Addiction, (Baltimore, MD: CMCS Informational Bulletin, January 2016), https://www.medicaid.gov/federal-policy-guidance/downloads/cib-02-02-16.pdf. ↩︎
- Several states mentioned plans to implement quantity limits based on a “morphine equivalent dose” (MED), which is the amount of opioid prescription drugs, converted to a common “standard” unit (milligrams of morphine). For example, both 60 mg of oxycodone (approximately 2 tablets of oxycodone sustained-release 30 mg) and approximately 20 mg of methadone (4 tablets of methadone 5 mg) are equal to 90 MMEs (morphine milligram equivalents). ↩︎
- “Clinical edits” are clinically-based claims adjudication rules that a claims system will follow when processing a pharmacy claim. ↩︎
- Step therapy prior authorization criteria involves requiring the use of another agent prior to the use of a specific opioid. ↩︎
- Prescription Drug Monitoring Programs (PDMPs) are state-run electronic databases that are valuable tools for addressing prescription drug diversion and abuse. Currently, except for Missouri, every state and the District of Columbia operates a PDMP. On July 17, 2017, however, Missouri Governor Eric Greitens issued an Executive Order directing the Missouri Department of Health and Senior Services (DHSS) to implement a PDMP and promulgate regulations to require dispensers to submit controlled substance prescription and dispensation information to DHSS or its designee. ↩︎
- Lock-in programs limit Medicaid beneficiaries to a specific pharmacy and/or prescriber. These programs are intended to prevent Medicaid beneficiaries from obtaining excessive quantities of prescribed drugs through visits to multiple physicians and pharmacies. ↩︎
- “Opioid Overdose Reversal with Naloxone (Narcan, Evzio),” National Institute on Drug Abuse, revised September 2016, https://www.drugabuse.gov/related-topics/opioid-overdose-reversal-naloxone-narcan-evzio. ↩︎
- Ravi Gupta, Nilay Shah, and Joseph Ross, “The Rising Price of Naloxone – Risks to Efforts to Stem Overdose Deaths,” New England Journal of Medicine, 375 (December 2016): 2213-2215, http://www.nejm.org/doi/full/10.1056/NEJMp1609578#t=article. ↩︎
- “Medication-Assisted Treatment (MAT),” Substance Abuse and Mental Health Services Administration, November 2016, https://www.samhsa.gov/medication-assisted-treatment. ↩︎
- The Pew Charitable Trusts, Medication-Assisted Treatment Improves Outcomes for Patients With Opioid Use Disorder, (Washington, DC: The Pew Charitable Trusts, November 2016), http://www.pewtrusts.org/en/research-and-analysis/fact-sheets/2016/11/medication-assisted-treatment-improves-outcomes-for-patients-with-opioid-use-disorder. ↩︎
- Colleen Grogan et al., “Survey Highlights Differences in Medicaid Coverage for Substance Use Treatment and Opioid Use Disorder Medications,” Health Affairs 35 no. 12 (December 2016): 2289-2296, http://content.healthaffairs.org/content/35/12/2289. ↩︎
- AR and IL did not respond to the MAT drug coverage question; however, a Health Affairs article (citation below) that uses 2013-2014 data indicates that all 51 states cover buprenorphine. Colleen Grogan et al., “Survey Highlights Differences in Medicaid Coverage for Substance Use Treatment and Opioid Use Disorder Medications,” Health Affairs 35 no. 12 (December 2016): 2289-2296, http://content.healthaffairs.org/content/35/12/2289. ↩︎
- National Association of Medicaid Directors, NAMD Statement on Graham-Cassidy, (Washington, DC: NAMD Press Release, September 2017), http://medicaiddirectors.org/category/press-release/. ↩︎
- Thomas Price and Seema Verma letter to governors, March 14, 2017, https://www.hhs.gov/sites/default/files/sec-price-admin-verma-ltr.pdf. ↩︎
- Medicaid work requirement proposals generally require beneficiaries to verify their participation in approved activities, such as employment, job search, or job training programs, for a certain number of hours per week to receive health coverage. The proposals typically would exempt certain populations. To date, CMS has not approved state waiver requests to require that Medicaid beneficiaries work as a condition of eligibility. ↩︎
- Implementation dates for Section 1115 waiver provisions included/cited in this report are the dates proposed in state waiver applications submitted to CMS. ↩︎
- Elizabeth Hinton, MaryBeth Musumeci, Robin Rudowitz, and Larisa Antonisse, Section 1115 Medicaid Demonstration Waivers: A look at the Current Landscape of Approved and Pending Waivers, (Washington, DC: Kaiser Family Foundation, September 2017), https://modern.kff.org/medicaid/issue-brief/section-1115-medicaid-demonstration-waivers-a-look-at-the-current-landscape-of-approved-and-pending-waivers/. ↩︎
- Kaiser Family Foundation, Proposed Changes to Medicaid Expansion in Kentucky (Washington, DC: Kaiser Family Foundation, August 2017), https://modern.kff.org/medicaid/fact-sheet/proposed-changes-to-medicaid-expansion-in-kentucky/. ↩︎
- Beginning at 5 hours/week and increasing to a maximum of 20 hours/week. ↩︎
- Matthew Bevin, Kentucky Health: Helping to Engage and Achieve Long Term Health, (KY Office of the Governor, August 2016), https://www.medicaid.gov/Medicaid-CHIP-Program-Information/By-Topics/Waivers/1115/downloads/ky/ky-health-pa.pdf. ↩︎
- Commonwealth of Massachusetts Office of Medicaid, MassHealth Section 1115 Demonstration Amendment Request, (Commonwealth of Massachusetts Office of Medicaid, September 2017), https://www.medicaid.gov/Medicaid-CHIP-Program-Information/By-Topics/Waivers/1115/downloads/ma/ma-masshealth-pa3.pdf. ↩︎
- MaryBeth Musumeci, Elizabeth Hinton, and Robin Rudowitz, Proposed Medicaid Section 1115 Waivers in Maine and Wisconsin (Washington, DC: Kaiser Family Foundation, updated August 2017), https://modern.kff.org/medicaid/issue-brief/proposed-medicaid-section-1115-waivers-in-maine-and-wisconsin/. ↩︎
- In 2014, Wisconsin implemented a new Section 1115 waiver covering childless adults ages 19 to 64 with income up to 100% FPL; those above 100% FPL are covered in the Marketplace. (Wisconsin covers childless adults without ACA enhanced matching funds.) ↩︎
- State proposes to exempt childless adults ages 19 to 49 from the 48 month time limit if working or attending job training 80 hours per month and proposes to use Medicaid funds to offer job training as a covered benefit for childless adults. ↩︎
- State of Wisconsin Department of Health Services, BadgerCare Reform Demonstration Project: Coverage of Adults Without Dependent Children with Income at or Below 100 Percent of the Federal Poverty Level, Section 1115 Demonstration Waiver. Amendment Application, (State of Wisconsin Department of Health Services, June 2017), https://www.medicaid.gov/Medicaid-CHIP-Program-Information/By-Topics/Waivers/1115/downloads/wi/wi-badgercare-reform-pa.pdf. ↩︎
- Alaska Department of Health and Social Services, Alaska Behavioral Health Reform 1115 Waiver Concept Paper (Alaska Department of Health and Social Services, January 2017), http://dhss.alaska.gov/HealthyAlaska/Documents/Initiatives/1115_ConceptPaper1-5-17wAppendix.pdf. ↩︎
- New Mexico Human Services Department, Centennial Care 2.0: Section 1115 Demonstration Waiver Renewal Concept Paper, (New Mexico Human Services Department, May 2017), http://www.hsd.state.nm.us/uploads/files/CC%202%200%20Concept%20Paper_FINAL.pdf. ↩︎
- Kaiser Family Foundation, 50-State Medicaid Budget Survey Archives, (Washington, DC: Kaiser Family Foundation, October 2017), https://modern.kff.org/medicaid/report/medicaid-budget-survey-archives/. ↩︎
- State fiscal years begin July 1 except for these states: NY on April 1; TX on September 1; AL, MI and DC on October 1. ↩︎