Medicaid Coverage of Family Planning Benefits: Results from a State Survey
Jenna Walls, Kathy Gifford, Usha Ranji, Alina Salganicoff, and Ivette Gomez
Published:
Executive Summary
An updated version of this report was released on February 17, 2022 and can be found here.
Overview
Medicaid plays a major role financing family planning services for low-income women in the United States. Family planning services are “mandatory” benefits under Medicaid and must be provided to individuals of childbearing age free of cost-sharing. There is, however, no formal federal definition of “family planning,” which has given states considerable discretion to determine the specific services covered under this benefit. Furthermore, a state may establish different coverage requirements for Medicaid funded family planning services for different eligibility pathways. The Affordable Care Act (ACA) created a new Medicaid eligibility category which has federally-specified coverage requirements for aspects of family planning (contraceptives, screening services, and counseling), but these requirements do not apply to traditional Medicaid available prior to the ACA. This has magnified the potential for variations in coverage standards for different Medicaid eligibility pathways (e.g. traditional Medicaid available prior to the ACA, ACA Medicaid expansion, or Medicaid Family Planning Expansion program) within a state. The multiple pathways and coverage options make it difficult to assess coverage differences for family planning services both within and across states under fee-for-service.
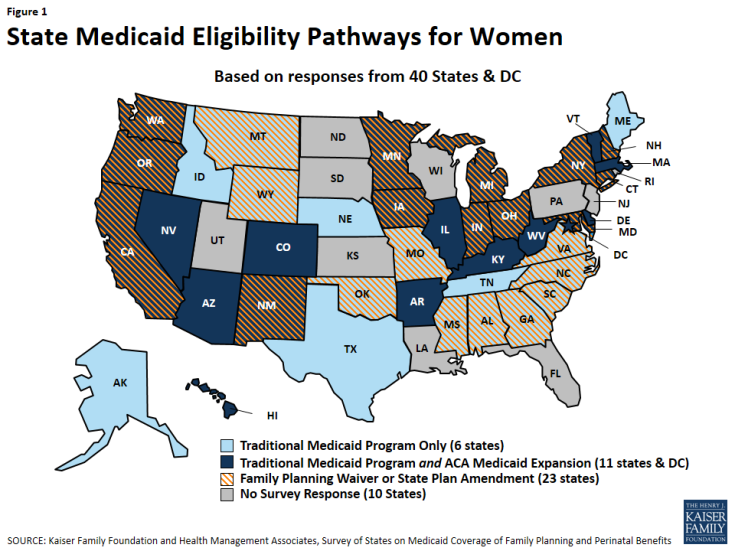
This report presents findings from a state-level survey on states’ family planning benefits under Medicaid, as of July 2015. The survey queried states about their coverage policies under fee-for-service for the following family planning services: reversible contraceptives, sterilization services, fertility diagnosis and treatment, services related to family planning and sexual health such as cancer treatment and partner violence, and managed care policies. The survey identifies differences between states as well as within states between Medicaid eligibility pathways: traditional Medicaid (available pre-ACA), Medicaid expansion under the ACA, and family planning-only coverage through a state Medicaid waiver or State Plan Amendment (SPA). All 50 states and the District of Columbia were invited to respond to the survey, but data are presented for 40 states and the District of Columbia that provided responses (Figure 1). Throughout the report, DC is counted as a state, totaling 41 respondents.
Key Findings
Reversible Contraception
All responding states cover nearly all prescription contraceptive methods approved by the Food and Drug Administration under their fee-for-service programs, including IUDs and implants (Table 1).1 Coverage of over-the-counter contraceptives, particularly emergency contraception, showed more variation and utilization controls. Most states, but not all states, have aligned their coverage of prescription contraceptives across all of their Medicaid eligibility pathways.
- Thirty-six out of 41 states covered all prescription methods in the survey under their traditional Medicaid pathway. Of the five states that did not cover all methods, two states did not cover one form of injectable and three of them did not cover ella, an emergency contraceptive pill.
- While most contraceptives are covered, a number of states apply utilization controls such as quantity limits on oral contraceptives and injectables. Some states, however, have moved in the opposite direction, permitting clinics to dispense a 12-month supply of oral contraceptives.
- Coverage of IUDs and implants is widespread and no states reported that they limited access to long-acting reversible contraceptives (LARCs) by requiring prior authorization, although some have utilization limits under fee-for-service, such as limiting coverage to certain brands.
- States are considering and adopting a variety of payment policies to facilitate postpartum LARC While maternity services are typically paid for with a global fee that includes postpartum care, some states have developed a separate payment outside the global fee to compensate clinicians and hospitals for postpartum LARC insertions. Several states continue to include either the device or clinician fee in the maternity global fee, which can be a disincentive for providers to insert postpartum LARCs given the relatively higher costs of IUDs and lack of separate reimbursement for the insertion.
| Table 1: Summary Findings on State Coverage of Contraceptive Methods in Traditional Medicaid Programs | |
| Covers 20 forms of prescription contraceptives in Traditional Medicaid Program (36/41 states) | AK, AR, AZ, CO, CT, DC, DE, GA, HI, IA, IL, IN, KY, MA, MD, MI, MN, MO, MS, MT, NC, NE, NH, NM, NV, NY, OH, OK, OR, TN, TX, VA, VT, WA, WV, WY
5 states that do not cover all methods: CA, ME cover all methods except Injectable- subcutaneous AL, ID, SC cover all methods except ella emergency contraceptive |
| Cover 3 forms of LARC in Traditional Medicaid Program (41/41states) | AL, AK, AZ, AR, CA, CO, CT, DE, DC, GA, HI, ID, IL, IN, IA, KY, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NM, NY, NC, OH, OK, OR, SC, TN, TX, VT, VA, WA, WV, WY |
| Covers 2 forms of Emergency Contraception in Traditional Medicaid Program (35/41 states) | AK, AR, AZ, CA, CO, CT, DC, DE, GA, HI, IA, IL, MA, MD, ME, MI, MN, MO, MT, NC, NE, NH, NM, NV, NY, OH, OK, OR, TN, TX, VA, VT, WA, WV, WY |
| Covers 4 forms of OTC contraceptives Traditional Medicaid Program (22/41 states) | AK, AZ, CA, DC, HI, IA, IL, MA, MD, MI, MN, MT, NE, NH, NM, NV, NY, OH, OK, VA, WA, WY |
| NOTES: Prescription contraceptive methods in this survey are: Copper IUD, Hormonal IUD, Implant, Injectable- intra-muscular, Injectable- subcutaneous, Diaphragm, Contraceptive Patch, Vaginal Ring, Oral Contraceptive Pills Combined, Oral Contraceptive Pills- Progestin Only, Oral Contraceptive Pills-Extended Use, ella Emergency Contraceptive Pills, Tubal Ligation- General, Tubal Ligation- Post Partum, Sterilization Implant, and Vasectomy. LARC methods in this survey are: Copper IUD, Hormonal IUD, and Implant. OTC contraceptive methods in this survey are: Male condom, spermicide, sponges and levonorgestrel emergency contraceptive pills. | |
- Coverage for emergency contraception (EC) pills, particularly the over-the-counter (OTC) product (levonorgestrel, also known as Plan B), is not as uniform as for the prescription method (ulipristal acetate, also known as ella). While at least one form of EC pills is covered in traditional Medicaid programs in most states, the OTC option is covered in fewer states and subject to greater utilization controls, sometimes requiring a prescription. Three states report that they do not cover either type of EC pills. All states reported that they cover the copper IUD, which can be used as an EC, in all of their pathways.
- Variation in coverage across the states was most notable for over-the-counter (OTC) contraceptives, including condoms and Plan B emergency contraception. Coverage for OTC supplies also varied across state Medicaid eligibility pathways, and a number of states require prescriptions for coverage, which creates an access barrier for products the FDA has deemed to be safe and effective for over-the-counter use.
Sterilization and Fertility Services
Most states cover sterilization services in their FFS program, but few pay for fertility services. Federal law specifies that states must cover surgical and implant sterilization procedures for women under ACA Medicaid expansion, and all of the responding states reported that they cover these procedures in traditional Medicaid as well.
- Medicaid family planning expansion program do not always pay for sterilization services for women.
- While all states reported they cover vasectomies under traditional Medicaid, not all cover the procedure in their family planning expansion programs or under their full scope Medicaid expansion programs.
- Very few states cover diagnostic testing related to fertility, including laparoscopy for women and semen analysis for men.
- Only one state covers fertility treatments for either women or men, but this is restricted to individuals who have infertility as a symptom of separate medical problem.
Family Planning-Related Services
The definition of high quality family planning encompasses a broad array of services including screening and treatment for cervical and breast cancers, interpersonal violence screening and prevention, and sexual health counseling. These family planning-related services, however, are less consistently covered by family planning expansion programs than contraceptives.
- Although breast cancer screening is considered “optional” under traditional Medicaid, it is a required benefit in ACA Medicaid expansion programs. All responding states provided breast cancer screening services under these two full scope eligibility pathways. Few states, however, provide this benefit through their family planning waiver or SPA.
- All states cover Pap screening for cervical cancer regardless of eligibility pathway, but follow-up tests for abnormal screening results are less likely to be covered in state family planning waivers or SPAs.
- HPV vaccines for young adults are covered in all but one state, but the benefit is less likely to be covered through a family planning expansion program.
- Contraceptive counseling and screening for intimate partner violence are covered by most states, but services are typically subject to restrictions and are not always covered for all eligibility pathways available within a state.
Managed Care Policies
The majority of states have capitated managed care contracts that include family planning services. Many of these states, however, do not address how utilization controls can be used in the context of family planning in their contracts. Some of the states noted that they contract with MCOs that include providers with religious objections to family planning in their networks, but not all of these states detailed referral processes to assure that women can get family planning care from other providers.
- Most of the responding states have capitated contracts that include family planning in the capitation rate. Just over one-third of these states explicitly address potential utilization controls on family planning services in the contracts with managed care organizations.
- A handful of states reported that they do not claim the enhanced 90% federal match for family planning services provided through managed care organizations.
- California and New York, states with the most beneficiaries, also contract with faith-based plans that oppose some forms of contraception. While California reported that they have a process in place for referral for family planning services for the beneficiaries in these plans, New York did not report a referral practice.
Conclusion
The analysis of state responses to this survey found that overall most states cover a broad range of prescription contraceptive methods in their full scope, traditional Medicaid programs and their full scope ACA Medicaid expansions, but finds more variation in coverage through the family planning expansion programs. Thirty-six of 41 surveyed states report that they cover all prescription contraceptives for women through their full scope programs. While states are not required to cover all methods under all pathways, most do. However, there is more variation between and within states for coverage of over-the-counter contraceptives, including condoms and Plan B emergency contraception pills. In some states that provide coverage, it is only with a prescription which can limit access to these safe and effective methods. Furthermore, over the years the family planning field has evolved to encompass other services beyond contraception that help women and men maintain and control their reproductive and sexual health. Medicaid coverage for prevention and management of breast and cervical cancers and screening for interpersonal violence is available in most states, but not as consistently as for contraceptives. These preventive services must be covered by new private insurance plans as a result of the ACA, but there is no requirement that they be covered under traditional Medicaid or under the family planning expansion programs.
Access to the full range of contraceptive methods as well as related family planning services has become a standard of comprehensive health care for women and men in their reproductive years.2 As enrollment in the Medicaid program continues to grow as a result of the ACA and state decisions to expand coverage for family planning services, policy choices defining coverage of services under Medicaid family planning will continue to be a significant force shaping access to sexual and reproductive health services for low-income women and men in years to come.
Acknowledgements
The authors express appreciation for the assistance of several individuals who assisted with the preparation, testing, and refinement of the survey instrument, including Yali Bair of Ursa Consulting, Amy Moy from the California Family Health Council, Tasmeen Weik of the federal Office of Population Affairs, Melanie Reece of Colorado’s Department of Health Care Policy and Financing, and Lisa DiLernia of Michigan’s Department of Health and Human Services.
We thank the following colleagues from Health Management Associates: Joan Henneberry for guidance and subject matter expertise; Dennis Roberts for database development and management; and Nicole McMahon for assistance with compiling the state data tables.
We also thank the Medicaid directors and staff in the 40 states and the District of Columbia who completed the survey on which this report is based.
Introduction
Introduction
Overview
Medicaid is the primary funding source for family planning services for low-income women. While the federal and state governments jointly finance the program, states operate their programs and establish benefits, eligibility and coverage policies subject to broad federal guidelines. To understand variations in the scope of coverage for family planning and perinatal services and related state Medicaid policies across the nation, the staff of the Kaiser Family Foundation and Health Management Associates surveyed states about benefit policies in place as of July 1, 2015, for family planning and perinatal services.
The survey was conducted between October 2015 and February 2016. Forty states and the District of Columbia responded to the survey. Non-responding states are: Florida, Kansas, Louisiana, New Jersey, North Dakota, Pennsylvania, Rhode Island, South Dakota, Utah and Wisconsin. A majority of the states responding to the survey contract with managed care organizations (MCOs) under a capitated structure to deliver Medicaid services, including family planning. While the survey asked states to consider only state Medicaid policies under fee-for-service when responding to the questions, it is possible that MCOs provide additional family planning services to enrollees as added value benefits, unless they are contractually prohibited from doing so.
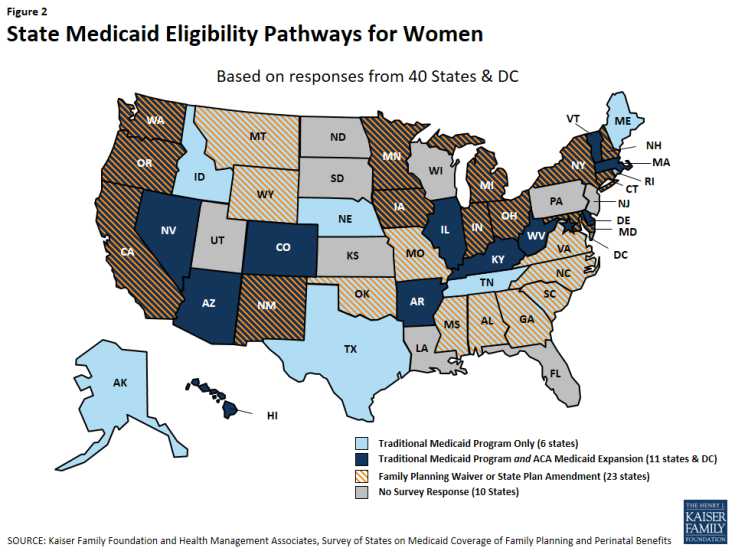
As illustrated in Figure 2, of the 41 responding states, as of July 1, 2015:
- 25 had adopted an ACA Medicaid expansion program 1
- 23 had a family planning-only waiver or SPA2
- 13 had both an ACA expansion program and a family planning-only waiver or SPA program
- 6 provide services through their traditional Medicaid program only, although one (Texas) also has an exclusively state-funded family planning program.
This report presents the survey findings on 41 states’ Medicaid coverage of family planning services as of July 2015 (DC is referred to as a state throughout this report, for simplicity). Summary tables are presented throughout the report and more detailed, state-level tables are in Appendix A. A companion report summarizing state Medicaid coverage of perinatal services will be issued in the near future.
Background
The manner in which family planning services are financed and organized is unique within the Medicaid program. Federal law:
- Classifies family planning services and supplies as a “mandatory” benefit category that states must cover, but states have some discretion as to which services they include in this category;
- Prohibits copayments or any other form of patient cost sharing for family planning services;
- Entitles beneficiaries to seek family planning services from any provider that participates in the Medicaid program, called freedom of choice; and
- Provides a 90% federal matching rate for the costs of family planning services, a higher proportion than for other services. States pay the remaining 10% of costs.
In addition to long-standing coverage under traditional Medicaid programs, states have also applied to the federal government via “Section 1115 Medicaid waivers” establish programs that extend Medicaid coverage for family planning services only to women and men who are not otherwise eligible for full-scope Medicaid. Prior to the ACA, waivers were the only mechanism for states to establish these limited scope programs. The waivers must be approved by the Centers for Medicare and Medicaid Services (CMS), the federal agency that oversees the Medicaid program. Multiple studies have found that these programs prevent unintended pregnancies and abortions, thus improving women’s health and saving money for the federal and state governments.3
The ACA made many changes to Medicaid coverage that affected beneficiaries’ access to family planning services under the program. Beginning in 2014, states could expand Medicaid eligibility to most people with incomes below 138% FPL without regard to categorical eligibility. As of March 14, 2016, 31 states and the District of Columbia have chosen to adopt full scope Medicaid expansion programs.4 ACA also defined a minimum “Alternative Benefit Plan” (ABP) that states must provide to beneficiaries under this full scope Medicaid expansion option. The ACA specifies that the ABP must include ten “essential health benefits,” including preventive services, at no cost to the patient.5 The preventive services under this policy include several services related to family planning, such as all FDA-approved contraceptives with a prescription, testing for sexually transmitted infections, screening for cervical and breast cancers, the HPV vaccine, and screening for intimate partner violence. Traditional, pre-ACA Medicaid programs and waiver-specific programs, however, are not bound by the ABP requirements, which means that the benefits package can vary within states for different Medicaid populations based on their eligibility pathway (Appendix B).
The ACA also eased the process for states to establish limited scope family planning programs, enabling them to change their program by adopting “state plan amendments,” or SPAs, without requesting a federal waiver of Medicaid rules. States could continue to operate their family planning programs under the waivers as they had historically done, or they could establish the program under SPA authority.
| Table 2: Medicaid Eligibility Pathways for Family Planning Services |
| Traditional Medicaid – Medicaid coverage available prior to the Affordable Care Act (ACA) was based on an individual having income below states’ very low thresholds as well as being in one of the program’s eligibility categories: pregnant, mothers of children 18 and younger, disabled, or over age 65 |
| ACA Medicaid Expansion – The ACA allowed states to eliminate categorical requirements and extend Medicaid to most women and men with family income at or below 138 percent of the federal poverty level. |
| Family planning-only programs – Several states operate programs that provide Medicaid coverage just for family planning services to women who are not otherwise eligible for full Medicaid benefits. These programs are authorized either with waivers or State Plan Amendments (SPAs) that must be approved by CMS. |
There is a minimum floor of family planning services that must be covered under ACA Medicaid expansion, but that is not the case for traditional Medicaid or family planning-only coverage (Table 2). This survey asked states about the scope of states’ coverage of family planning benefits under multiple eligibility pathways for Medicaid. Detailed findings from 40 states and DC on commonalities and differences between and within states are presented for reversible contraceptives, sterilization services, fertility diagnosis and treatment, related sexual health services such as cancer treatment and partner violence screening, and managed care policies.
Reversible Contraception
| Key Finding: Prescription Contraceptives |
| All responding states provide coverage for most prescription contraceptive methods approved by the Food and Drug Administration (FDA). In addition, with few exceptions, most states aligned their coverage of prescription contraceptives across all of their Medicaid eligibility pathways.
While most contraceptives are covered, a number of states apply utilization controls such as quantity limits on oral contraceptives and injectables. |
The survey examined state coverage policies for multiple types of contraceptive devices and methods including, prescription and non-prescription methods, as well as reversible methods and sterilization procedures for women and men. Reversible methods include a wide range such as long-acting reversible contraceptives (LARCs) – intra-uterine devices (IUDs) and implants; oral contraceptives; injectables, emergency contraceptives; and various other products and devices available by prescription or over-the-counter (OTC).
Long-Acting Reversible Contraceptives (LARCs)
| Key Finding: LARC |
| Coverage of IUDs and implants is widespread and no states limited access to LARCs by requiring prior authorization or other utilization limits. |
LARCs are highly effective for extended periods of time, ranging from three to 10 years depending on the specific type that a woman uses. In the United States, three types of LARCs are available: Hormonal and non-hormonal IUDs and implants. All states participating in the survey cover all LARC methods through all of their Medicaid programs offering family planning services (Table 3). The ACA requires coverage of these benefits in Medicaid expansion programs.
| Table 3: State Coverage of LARCs | ||||
| Contraceptive Device or Service | Traditional Medicaid (n = 41) |
Family Planning Waiver or SPA (n = 23) |
ACA Medicaid Expansion (n=25) |
Apply Limitations or Restrictions |
| Copper IUD | 41 | 23 | Required | 3 |
| Hormonal IUD | 41 | 23 | Required | 6 |
| IUD Removal | 41 | 23 | Required | 4* |
| Implant | 41 | 23 | Required | 3 |
| Implant Removal | 41 | 23 | Required | 3* |
| * “Medical necessity” is included in the count of states with restriction or limitations for IUD and implant removal. | ||||
LARC Limitations or Restrictions
Only a few states noted utilization controls or restrictions for devices. Typically, restrictions apply to benefit frequency or for specific devices (Table 4). For device removal, “medical necessity” was treated as a limitation since it is not clear whether states consider the desire to become pregnant a medical necessity. This was the most common restriction noted for device removal.
For removal of an IUD, Alabama specified the following criteria: the recipient develops high blood pressure or other medical condition that would allow for a progestin only method; any nulliparous woman who has a spontaneous expulsion within six months of placement; Mirena IUD is removed to allow a pregnancy; surgical removal of an embedded IUD in an office or outpatient setting. Missouri noted that the specific CPT code for IUD removal is not covered, but providers are instructed to use an office visit code for reimbursement. In addition to limits on quantity, California noted that LARC devices are limited to clinic dispensing only.
| Table 4: State Utilization Controls for LARCs | ||||
| IUD Device | IUD Removal | Implant Device | Implant Removal | |
| Quantity/frequency limits | AL, CA, MI, MO | CA, MI, MO | ||
| Brand/type restrictions | AL, ME, OK | |||
| Coverage subject to certain medical conditions or “medical necessity” | AL, NY, OK, TN | NY, OK, TN | ||
| Removal covered to allow pregnancy | AL | |||
LARCs Provided Immediately Post Labor and Delivery
| Key Finding: LARC Reimbursement |
| States are experimenting with a variety of payment policies to facilitate postpartum LARC insertion. Approaches include separate payments for LARC device as well as clinician fee outside of the global fee. Several states continue to include either the device or the clinical fee within the global fee that is used to typically pay for maternity care. |
States were asked how they reimburse providers for LARCs provided immediately after labor and delivery. This has been the source of considerable research, public health education, and policy activity in recent years in part because data that suggests birth spacing is an important component of healthy pregnancies and optimal birth outcomes.1 LARCs are among the most effective forms of reversible contraception and research suggests that providing them during the postpartum period, either at the time of delivery or at the follow up visit can help lower the rate of unintended pregnancies.
Labor and delivery is typically reimbursed through a global fee that covers the costs of all maternity care, including labor and delivery services, and postpartum care. Many providers have reported that the global fee is not sufficient to cover the costs of providing a LARC postpartum at the time of delivery or at the follow up postpartum visit. The absence of a separate fee or an increase has been a disincentive for some providers. Among the 41 states responding to the survey, the reimbursement methodology for hospitals most frequently reported is a global fee for both the LARC device and insertion (17 states), followed by 10 states that include insertion in a global fee and reimburse hospitals separately for the device. Conversely, the most frequently reported methodology for reimbursing other providers was a separate fee for both the device and insertion (25 states). Table 5 illustrates summary data for reimbursement for LARC device and insertion post labor and delivery. Appendix Table A1 provides detail for each state response.
Three states indicated no specific hospital reimbursement for post labor/delivery LARCs. Arizona indicated that when the state implemented a hospital APR DRG2 payment methodology, the fee associated with the labor and delivery for the hospital did not consider the inclusion of LARC, but it will be considered in the future. The District of Columbia noted it does not reimburse for immediate post- partum LARC, but the managed care plans do, and the reimbursement methodology varies across the three contracted health plans. North Carolina reported that many hospitals and physician offices have chosen not to place LARCs in the immediate postpartum period.
| Table 5: Reimbursement Structure for Post Labor & Delivery LARC Device and Services | ||
| Hospital (n=41) |
Other Provider (n=40)* |
|
| Global Fee includes LARC Device and Insertion | 17 | 6 |
| Separate Fee for Both LARC Device and Insertion | 7 | 25 |
| Device included in Global Fee; Insertion Separate Reimbursement | 4 | 4 |
| Device Separate Reimbursement; Insertion included in Global Fee | 10 | 1 |
| Other | 3 | 4* |
| *Maryland did not provide a response for reimbursing other providers; Delaware noted that other providers are reimbursed for insertion as part of a global fee, but did not provide a response for the device itself. | ||
States were asked to identify and briefly describe any known policy or reimbursement barriers that inhibit the provision of LARCs immediately following labor and delivery. Several states that use a global fee to reimburse hospitals noted low utilization of postpartum LARC provision, whether the global fee was for the device, insertion or both. Several states reported plans to change their reimbursement methodology.
CMS Bulletin on Payment Approaches for LARCs
In April 2016, CMS issued a bulletin addressing reimbursement for LARC devices. CMS identifies some of the barriers that have impeded broader use of these devices, including low reimbursement levels, the absence of a separate payment from the typical global maternity fee, and administrative hurdles for providers to keeping devices on hand so that they can be provided upon request. The bulletin highlights recent state activity to address these barriers as well as potential strategies to encourage clinicians to provide LARCs postpartum and in the primary care setting, including:
- Raising provider payment rates
- Unbundling payment for LARC from other maternity services.
- Reducing logistical barriers for supply management
- Removing administrative barriers for provision of LARC
SOURCE: Centers for Medicare and Medicaid Services (2016). State Medicaid Payment Approaches to Improve Access to Long-Acting Reversible Contraception.
Colorado and Tennessee use the global maternity fee to reimburse hospitals for both the device and insertion. Colorado notes that postpartum LARCs are currently covered and available under normal labor and delivery reimbursement but also stated that the state is “looking to carve out payment from the global [labor and delivery] fee, but [are] still working on an appropriate process and CMS approval.” Tennessee plans to unbundle payments in 2016 to allow for separate reimbursement for postpartum LARCs “…in hopes of improving access for this contraceptive technology to Medicaid enrollees.”
Georgia reimburses hospitals for LARC insertion using a global fee and reimburses for the device separately, but notes that few LARCs have been purchased by hospitals to date for immediate postpartum insertion. The state intends to make system changes in 2016 that will allow separate reimbursement to hospitals for postpartum LARC insertion.
Texas included LARC device reimbursement within the hospital DRG but effective January 1, 2016 allows hospitals to receive reimbursement for the LARC device in addition to the DRG payment when the device is inserted immediately postpartum.
Other states reported issues with global payments for postpartum LARC procedures and devices: Massachusetts, which uses global payments, noted that providers would prefer a different reimbursement mechanism; Minnesota noted that they do not have a different DRG for LARC procedures/devices following a birth, as there is with sterilization procedures following a birth; Oregon reported that the DRG payment does not adequately cover the hospital cost so they are currently considering options to enhance the reimbursement amount; Washington changed its policy to allow separate reimbursement for a LARC device and enhanced reimbursement for LARC insertion added to the RVU-based fee.
Two states that use a global hospital reimbursement for both the device and LARC insertion, Michigan and Ohio, reported no barriers to utilization. Virginia, which also uses a global reimbursement methodology, described a four month pilot project implemented by one of its managed care plans that allowed for separate payment for the LARC device. However, there were no requests for immediate postpartum LARC insertions, so the pilot ended. The state noted “…it appears that a primary potential barrier of accessing this method of LARC is lack of provider and member education and training versus policy and reimbursement.”
Oral Contraceptives
| Key Finding: Oral Contraceptives |
| All responding states cover daily use oral contraceptives (Combined, Progestin only, as well as Oral Extended and Continuous use) regardless of the eligibility pathway.
Few states allow a 12-month supply for oral contraceptives; supply limits are the most common method to control utilization. |
Oral contraceptives are the most commonly used form of reversible contraception among women in the United States. All states surveyed cover all forms of oral contraceptives in their traditional Medicaid program and their family planning waiver or SPA as applicable. The ACA requires states to cover oral contraceptives for adults in their Medicaid expansion programs (Table 6). The survey also asked states whether they provide coverage for 12 months’ supply of oral contraceptives. Eleven of the responding states indicated that they allow a 12-month supply of oral contraceptives to be dispensed, but typically with some restrictions (7 states). For example, California3 and Virginia noted that 12-month dispensing is restricted to clinics and on-site dispensing by medical providers. Pharmacies may not dispense a 12-month supply, with the exception of California’s family planning expansion program.
| Table 6: Oral Contraceptive Coverage | |||
| Benefit | Traditional Medicaid (n=41) |
ACA Medicaid Expansion (n=25) |
Family Planning Waiver or SPA (n=23) |
| Oral Contraceptives (specific) | |||
| – Combined Estrogen Progestin | 41 | Required | 23 |
| – Progestin Only | 41 | Required | 23 |
|
– Oral Extended/Continuous Use
|
41 | Required | 23 |
Limitations and Restrictions
Supply limits are the most common restriction reported for oral contraceptives, and limits are most usually tied to the dispensing provider, or program (Table 7). Seven states reported they restrict their supply to three months. Vermont requires prior authorization for non-preferred brands, along with a trial of a preferred brand. Mississippi and Vermont include oral contraceptives on the state’s Preferred Drug List (PDL), which means that coverage for these drugs is not subject to prior authorization. Appendix Table A2 provides state response detail around 12 month supply dispensing and utilization controls for oral contraceptives.
| Table 7: State Utilization Policies for Oral Contraceptives | ||
| Utilization Control | States Utilizing | Notes |
| Limited to 3 month/90 day supply (7) | CA, IL, MI, MN, MS, NH, WY | CA and MI limit applies to pharmacy dispensing |
| Limited to 6 month supply (1) | CO | |
| Allow 12 month supply (11) | AL, CA, IN, MO, MS, NM, OR, SC, TX, VA, WA | AL, CA, MS, SC, TX, VA allowance applies to clinic dispensing only; OR applies only to family planning waiver service recipients |
| Quantity limit, unspecified (1) | AR | |
| Pharmacy benefit only (2) | GA, CA | CA limit applies only to extended use oral contraceptives for family planning expansion program |
| Included on Preferred Drug List (2) | MS, VT | |
| Prior Authorization (2) | CA, VT | CA limit applies to extended use oral contraceptives only; VT requires prior authorization for non-preferred brand with trial of preferred brand |
Other Prescription Contraceptives – Injectable, Diaphragm, Patch, Ring
Almost all of the responding states covered the remaining prescription contraceptives included in the survey across all available eligibility pathways. These methods include injectable contraceptives, the diaphragm, contraceptive patch, and vaginal ring. California and Maine indicated they do not cover the subcutaneous injectable, but California was in the process of adding coverage for this method.
Limitations and Restrictions
The most common utilization control noted by states for these contraceptive methods is limits on quantity or dose (Table 8). States that identified quantity and/or dose limits for one or more of the methods include: Alabama, Arkansas, California, Illinois, Michigan, and Missouri. Other restrictions pertain to the type of provider that can provide or dispense the contraceptive. California allows only clinics to dispense injectable contraceptives, but restricts dispensing of diaphragms to pharmacies. The place of service – either pharmacy or clinic – for the contraceptive patch and vaginal ring depends on the type of product dispensed. Michigan allows up to two diaphragms per year, but they must be dispensed from the same billing provider. States also reported other policies and program integrity measures. For example, in Texas the claim for the product must be accompanied by a family planning diagnosis code.
| Table 8: Coverage and Utilization Controls for Other Contraceptive Methods | ||||
| Traditional Medicaid (n=41) |
ACA Medicaid Expansion (n=25) |
Family Planning Waiver or SPA (n=23) |
Number of States with Utilization Controls | |
| Injectable – intra-muscular |
41 | Required | 23 | Quantity/dose: 5 Place of service: 1 Other: 4 |
| Injectable – subcutaneous | 39 | Required | 22 | Quantity/dose: 4 Other: 4 |
| Diaphragm | 41* | Required | 23 | Quantity/dose: 4 Place of Service: 2 Other: 1 |
| Contraceptive Patch | 41 | Required | 23 | Quantity/dose: 4 Place of Service: 1 Other: 3 |
| Vaginal Ring | 41* | Required | 23 | Quantity/dose: 3 Place of Service: 1 Other: 2 |
| * North Carolina covers diaphragm fitting only; Georgia covers the Vaginal Ring as a component of a family planning visit. | ||||
Emergency Contraceptives
| Key Finding: Emergency Contraception |
| At least one form of emergency contraceptive pills (levonorgestrel and ulipristal acetate) are covered in traditional Medicaid programs in all but three responding states. The over-the-counter option (levonorgestrel, also known as Plan B) is covered in fewer states and subject to greater utilization controls, including requiring a prescription. All states reported that they cover the copper IUD, which can be used as an emergency contraceptive, in all of their pathways. |
In addition to coverage for Copper IUDs, which can be used as an emergency contraceptive, the survey asked states about their policies with respect to oral emergency contraceptives, including ella (ulipristal acetate) which is only available with a prescription, and Plan B products (levonorgestrel)4 which are available OTC without a prescription.
A few states provide unrestricted access to emergency contraception, or provide Plan B without a prescription: Maryland, Nebraska, Oregon, and Washington. Only three states reported that they do not cover either form of emergency contraceptive pills in any of their Medicaid programs – Alabama, Idaho, and South Carolina. Thirty-eight states cover ella, and 35 cover both Plan B products and ella emergency contraceptives in their traditional Medicaid programs. The ACA requires that Medicaid expansion programs cover emergency contraceptives, but only with a prescription under the ABP (Table 9). This means that these programs must always cover ella, but only are required to cover Plan B with a prescription.
For OTC Plan B emergency contraception, 21 of 25 states with an ACA Medicaid expansion population provide coverage through that pathway. Alabama, Idaho, Indiana, Kentucky, Mississippi, and South Carolina do not provide emergency OTC contraceptive coverage in any of the eligibility pathways available within the state. Minnesota does not provide the benefit in its ACA Medicaid expansion, but does provide it in the traditional Medicaid program and through the family planning SPA. Wyoming does not provide the benefit through its family planning waiver but does cover the benefit under traditional Medicaid.
| Table 9 State Coverage of Emergency Contraceptives, by Type of Program | ||||
| Traditional Medicaid (n=41) |
ACA Medicaid Expansion (n=25) |
Family Planning Waiver or SPA (n=23) |
Number of States Noting Restrictions* | |
| Ella | 38 | Required | 20 | 7 |
| Plan B | 35 | 21 | 18 | 31 |
| Both Ella and Plan B | 35 | 21 | 17 | |
| Neither Ella nor Plan B | 3 | 0 | 2 | AL, ID, SC do not cover either ella or Plan B in traditional Medicaid; AL and SC do not cover in family planning waiver/SPA |
| * Requiring a prescription is not counted as a restriction for Ella, since a prescription is required in all states for this product. Requiring a prescription is counted as a restriction for Plan B products, which are available for purchase over the counter. | ||||
Utilization Controls and Restrictions for Emergency Contraceptives
In the case of ella, which requires a provider prescription, fewer states apply utilization controls across states compared to Plan B OTC products (Table 10). Only seven states note any kind of utilization control for ella, with quantity limits the most prevalent limitation. The most common utilization restriction imposed by states, other than requiring a prescription for OTC products is quantity control (6 states). Two states restrict the place of dispensing to pharmacies and Washington allows pharmacists to dispense OTC emergency contraceptives directly to the client without prescription. Appendix Tables A3 and A4 include more detail on state responses to survey questions concerning how states cover of emergency contraception.
Seven states cover Plan B products but do not cover other methods of OTC contraceptives (Georgia, Maine, Missouri, North Carolina, Tennessee, Vermont, and West Virginia). Of the 41 responding states, most require prescriptions for OTC products (29 states), including Plan B (27 states).
| Table 10: State Utilization Controls for Emergency Contraceptives | |
| Utilization Controls – Ella (Rx) | States with Utilization Control Policies |
| Quantity Limits (5) | AR, CA, IL, MN, NY, |
| Place of dispensing controls (1) | GA (pharmacy benefit only) |
| Gender controls (2) | AR, CA |
| On PDL (1) | MS |
| Utilization Controls – Plan B (OTC) | |
| Requires Prescription (27) | AK, AR*, AZ, CA, CO, CT, DC, DE, HI, IA, MA, ME*, MI, MO*, MT, NC*, NH, NM, NV, OH, OK, TN*, TX, VA, VT*, WV*, WY |
| Quantity Limits (6) | AR, CA, IL, ME, MN, NY, |
| Place of dispensing controls (2) | GA, TX (pharmacy benefit only) |
| Gender Controls (2) | AR, CA |
| Prior Authorization (1) | VT* |
| Age Controls (1) | GA |
| * Arkansas requires a prescription within the ACA Medicaid expansion population only. Georgia, Maine, Missouri, North Carolina, Tennessee, Vermont, and West Virginia do not cover OTC contraceptives except for Plan B emergency contraception. Vermont requires prior authorization for Plan B brand only. Other products are covered with a prescription. |
|
Over-the-Counter (OTC) Contraceptives
| Key Findings: OTC Contraceptives |
| There is more variation in coverage for over-the-counter contraceptive methods compared to prescription methods. A number of states also require prescriptions for these methods to be covered by Medicaid. |
In addition to emergency contraceptive options discussed above, states were asked about their Medicaid coverage policies for male condoms, spermicide and sponges which are available over the counter to the public. Under the ACA, full scope Medicaid expansion pathways must cover prescription methods, but the requirement does not apply to over the counter methods. The survey found more variability between states and between eligibility groups for over-the-counter methods, compared to prescription methods.
Fewer than half of responding states (20 states), cover all three types of OTCs in all eligibility pathways available within the state. Ten states do not cover any of the three OTC products in any Medicaid program: Alabama, Georgia, Idaho, Kentucky, Maine, Missouri, North Carolina, Tennessee, Vermont and West Virginia. As illustrated in Table 11, male condoms and spermicide are covered in 27 of the 41 responding states and the sponge in 26 states. All three types of contraceptives were covered for ACA Medicaid expansion groups in 18 states of the 25 states with that eligibility pathway. Most of the responding states with a family planning waiver or SPA cover male condoms, spermicide and sponges (18, 17, and 17 states respectively). Appendix Tables A5 and A6 provide additional detail on state coverage of OTCs and utilization controls applied and coverage of OTC contraceptives in each of the eligibility pathways.
| Table 11: Medicaid Coverage of OTC Contraceptives | ||||
| Traditional Medicaid (n=41) |
ACA Medicaid Expansion (n=25) |
Family Planning Waiver/SPA (n=23) |
Not Covered in Any Program | |
| Male Condoms | 27 | 18 | 18 | 11 |
| Spermicide | 27 | 18 | 17 | 12 |
| Sponges | 26 | 18 | 17 | 13 |
Utilization Controls for Over-the-Counter Contraceptive Supplies
Only six states noted no restrictions for any of the three OTC supplies for which they provide coverage: Illinois, Indiana,5 Maryland, Minnesota, Nebraska and Oregon (Table 12). Requiring a prescription is the most common utilization control for OTC contraceptive supplies. This is likely due, at least in part, to the established reimbursement mechanism to pharmacies for prescription drugs. Twenty-two states require a prescription or other form of documentation for OTCs. Michigan requires a prescription for spermicide and sponges purchased OTC, but does not require a prescription for condoms. Delaware also requires a prescription for both spermicide and sponge contraceptives, but does not cover male condoms.
Some states noted differences in coverage of OTCs based on whether the supply was obtained through a pharmacy benefit or through a clinic (point of service). For example, Virginia restricts access to all three OTC types to pharmacy dispensing only with a prescription. Connecticut does not cover spermicide under the retail pharmacy benefit (but does cover condoms that contain spermicide). Under the medical benefit, spermicide is covered when provided by an enrolled family planning clinic or from a medical/surgical supplier. Texas only allows reimbursement to family planning agencies for dispensing male condoms and spermicide. In Mississippi, condoms are only reimbursable through a medical claim for family planning waiver participants.
| Table 12: Methods Used by States to Restrict Utilization of OTC Contraceptive Supplies | |||
| Male Condom | Spermicide | Sponges | |
| No restrictions (6) | IL, IN, MD, MN, NE, OR | IL, IN, MD, MN, NE, OR | IL, IN, MD, MN, NE, OR |
| Quantity/Claim limits (3) | CA, MI, OH | CA, OH | CA |
| Point of service restriction (4) | MS, TX, VA | CT, TX, VA | VA |
| Prescription or other documentation required (22) | AK, AZ, AR, CA, CO, CT, DC, HI, IA, MA, MT, NV, NH, NM, NY, OH, OK, SC, VA, WA, WY | AK, AZ, AR, CA, CT, DE, DC, HI, IA, MA, MI, MT, NV, NH, NM, NY, OH, OK, SC, VA, WA, WY | AK, AZ, CA, CO, DE, DC, HI, IA, MA, MI, MT, NV, NH, NM, NY, OH, OK, SC, VA, WA, WY |
| Prior authorization (1) | OH | OH | |
| * Ohio requires prior authorization to exceed quantity limits (36/month for condoms;1/month spermicide) | |||
Sterilization Procedures
| Key Finding: Sterilization |
| Coverage of sterilization services varied by eligibility pathway. States must cover surgical and implant sterilization procedures for women under ACA Medicaid expansion, and all of the responding states reported that they cover these procedures in Traditional Medicaid as well. However, not all family planning expansion programs cover these services. |
This survey inquired about states coverage of sterilization procedures for women (tubal ligation and non-surgical essure) and men (vasectomy). As with FDA-approved reversible methods, the ACA requires coverage under the ACA Medicaid expansion to include surgical and non-surgical sterilization procedures for women. The requirement does not apply to vasectomy for men.
Federal Rules for Payment of Sterilization
Federal funds can only be used to pay for the sterilization of an individual when:
a) The individual is at least 21 years old at the time consent is obtained;
b) The individual is not a mentally incompetent individual;
c) The individual has voluntarily given informed consent in accordance with all the requirements prescribed in §§441.257 and 441.258; and
d) At least 30 days, but not more than 180 days, have passed between the date of informed consent and the date of the sterilization, except in the case of premature delivery or emergency abdominal surgery. An individual may consent to be sterilized at the time of a premature delivery or emergency abdominal surgery, if at least 72 hours have passed since he or she gave informed consent for the sterilization. In the case of premature delivery, the informed consent must have been given at least 30 days before the expected date of delivery.
Source: 42 CFR §441.253
The federal government requires states to cover sterilization procedures only when certain conditions are met. These requirements are intended to protect against coercive practices that had historically forced sterilizations upon marginalized groups, including low-income women, women with disabilities, women of color, and incarcerated women.1 Protections against these practices include requiring women to sign an informed consent form at least 30 days prior to a procedure as well as prohibition of federal matching funds for the sterilization of a mentally incompetent or institutionalized individual.
Most states with a Family Planning waiver or SPA also cover the procedures for women (Table 13), but there are exceptions. Ohio and Oregon do not cover tubal ligation (neither general nor post-partum) in their family planning programs. Connecticut, Georgia, Missouri, and Mississippi do not cover tubal ligation performed post-partum in their family planning programs, with Georgia noting that pregnant women are not enrolled in the state’s family planning waiver.
Although vasectomy is not a required benefit, all but two of the surveyed states covered this service for men. The District of Columbia and Hawaii, do not provide the benefit in their ACA Medicaid expansion programs. In Michigan, Missouri, Wyoming, Georgia and Maryland, only women are enrolled in their family planning waivers so they didn’t cover vasectomies. Ohio’s’ family planning SPA included men, but also did not cover vasectomies.
In the survey, three states noted utilization controls. North Carolina noted that only one procedure is allowed per lifetime. Kentucky requires prior authorization for vasectomies. Alabama will not pay for family planning services after a sterilization procedure has been conducted and also requires prior authorization for Essure.
| Table 13: Number of States Covering Sterilization Procedures, by Type of Program | |||
| Procedure | Traditional Medicaid (n=41) |
Family Planning Waiver/SPA (n=23) |
ACA Medicaid Expansion (n=25) |
| Tubal Ligation Post-Partum | 41 | 17 | Required |
| Tubal Ligation General | 41 | 21 | Required |
| Essure: Non-surgical | 41 | 19 | Required |
| Vasectomy | 41 | 17 | 23 |
Fertility Services
| Key Finding: Fertility Services |
| No state covers fertility treatments for either women or men. Some states cover diagnostic testing related to fertility, although some restrict the test for medical reasons other than for fertility. |
There are no federal requirements for state Medicaid programs to cover fertility testing or treatment such as medications, intrauterine insemination, or in-vitro fertilization for individuals enrolled in Medicaid. States may cover diagnostic services to detect the underlying medical reasons for infertility.
States were asked about diagnostic testing for both women (laparoscopy) and men (semen analysis). Nine of the 41 responding states cover fertility testing for women and men in their traditional Medicaid program as do 6 of the 25 responding ACA expansion states (Table 14). Just 3 of 23 states cover testing for women (Maryland, Minnesota, Oklahoma) and men (Alabama, Maryland, Minnesota) under a family planning waiver or SPA. Overall, five states provide the coverage for both genders in all of their eligibility pathways: Arkansas, Hawaii, Massachusetts, Maryland, and Nebraska. Notably, Nebraska is the only state that indicated it provides women with medication such as clomid and hCG, but only when infertility is a symptom of a separate medical problem. Appendix Tables A7 and A8 detail fertility testing policies for women and men.
| Table 14: Fertility Testing and Services in State Medicaid Programs, by Type of Program | |||
| Traditional Medicaid (n=41) |
ACA Medicaid Expansion (n=25) |
Family Planning Waiver/SPA (n=23) |
|
| Diagnostic Testing for Women | 9 | 6 | 3 |
| Diagnostic Testing for Men | 9 | 6 | 3 |
| Medications for women (Clomid, hCG) | 1 | 1 | 0 |
| Intrauterine Insemination | 0 | 0 | 0 |
| In-vitro Fertilization | 0 | 0 | 0 |
Family Planning Support Services
| Key Finding: Family Planning Support Services |
| There is broad coverage for some family planning-related services such as counseling and well woman visits, but considerably fewer states reported they cover screenings for intimate partner violence. |
Family planning services are an integral component of comprehensive primary, preventive, and sexual health care. The survey asked states about their coverage policies for family planning support services including contraceptive counseling, contraceptive follow-up and side effects management, well woman visits and check-ups, and intimate partner violence (IPV) screening. Nearly all states indicated that they cover support services, but this varied across eligibility pathways (coverage is required for ACA expansion programs), and some states indicated the service is not separately reimbursable, but is a component of some other type of office visit.
Coverage across Eligibility Pathways
Six states noted that contraceptive counseling is provided as a component of an office visit and is not separately reimbursable: Alaska, Alabama, Connecticut, Washington and West Virginia indicated the service is a component of a clinic or office visit and Georgia noted the service is a component of a family planning visit. In 2012, Illinois implemented a special initiative to pay an enhanced rate for family planning counseling services to select providers that have a family planning focus.
Coverage varied considerably across eligibility pathways for IPV screening (Table 15). Of the responding states with a family planning waiver or SPA, 16 out of 23 cover IPV screening. Nine states noted that intimate partner violence screening would be included in the context of other types of office visits or services. Maryland, Missouri, New Mexico, Virginia and Wyoming do not cover IPV screening in either their family planning waiver/SPA or in their traditional Medicaid program.
Limitations and Utilization Controls
A few states reported utilization controls for family planning support services. North Carolina allows one annual visit and six follow up exams for contraceptive counseling and follow up in its family planning waiver. Well woman visits are limited to one annual exam per year and IPV screening is left to the discretion of the provider. Alabama allows one initial visit, one annual visit and four periodic visits for contraceptive side effects management and for well woman visits. Contraceptive counseling is considered a component of these visits. The state also covers IPV screening for family planning waiver recipients receiving care coordination (not available under traditional Medicaid). Ohio provides one well woman or checkup visit per year.
| Table 15: Coverage for Family Planning Services | ||||
| Traditional Medicaid (n=41) |
Family Planning Waiver/SPA (n=23) |
Covered as a component of an office visit or other service |
Not Covered in Either Program | |
| Contraceptive Counseling | 40 | 22 | 6 | MD |
| Follow-up Visit/Side Effects Management | 41 | 22 | 1 | |
| Well Woman Visits/Check-ups | 41 | 22 | 4 | |
| Intimate Partner Violence Screening | 35 | 16 | 9* | MD, MO, NM, VA, WY |
| NOTES: *West Virginia’s Right from the Start program assesses each prenatal and infant client for partner or household violence. The state requires that the screening happen once, but the Designated Care Coordinator (DCC) is free to complete the assessment for partner violence at any time it is felt necessary. Service is billed under care coordination. The state also noted that screening is a component of a mental health assessment. Alabama provides screening under its care coordination benefit for family planning recipients. Oregon provides the service in its family planning waiver in the context of a contraceptive management visit. Connecticut, Illinois, Michigan, Montana, and Washington report that the service is provided with a clinic or routine office visit. Georgia noted the service is a component of a family planning visit. |
||||
Cervical and Breast Cancer Services
The survey asked states about their policies with respect to coverage of services for cervical and breast cancers under their traditional programs and family planning expansion programs. These include the HPV vaccine, pap smear and follow up testing after abnormal laboratory results, mammograms, genetic (BRCA) screening for high-risk women, and breast cancer preventive medication for high-risk women. These services are required coverage for the ACA expansion group as they are recommended by the US Preventive Services Task Force.
Cervical Cancer Services – PAP Test and Follow Up, HPV Vaccine
| Key Finding: Cervical Cancer Services |
| All states cover Pap screening for cervical cancer regardless of eligibility pathway, but follow-up tests for abnormal screening results are less likely to be covered in state family planning waivers or SPAs. Of the states that do provide coverage, many indicate that the procedures or services are covered as part of a family planning visit under a family planning waiver or SPA, rather than as a specific benefit. |
The survey inquired about coverage of pap and lab testing as well as additional screening procedures subsequent to an abnormal result from the pap test. These procedures include:
- Colposcopy is a procedure to examine the cervix following abnormal pap smear results. The procedure may include extracting a small sample of tissue (biopsy) during the examination.
- LEEP, Loop Electrosurgical Excision Procedure, is a treatment for abnormal cells on the cervix, using a thin wire loop that has an electric current to remove the abnormal tissue. LEEP may be performed after abnormal cells are discovered during a pap test, colposcopy or biopsy.
- HPV DNA testing consists of using one of various biologic tests on a sample of tissue to detect the presence of DNA or RNA from the human papilloma virus.
Coverage across Eligibility Pathways
All of the states responding to the survey provide coverage for pap smear and lab in all three Medicaid pathways. All states participating in the survey cover the three follow-up screening methods in their traditional Medicaid and ACA Medicaid expansion programs, except North Carolina, which does not cover HPV DNA testing through any Medicaid pathway offered in the state. Coverage of the three follow-up screening methods varies more in state family planning programs (Table 16). Five states do not cover any of the three follow-up procedures in their family planning waiver or SPA: North Carolina, Oklahoma, Oregon, Virginia and Washington. Four states cover HPV DNA testing within their family planning waiver or SPA, but do not cover colposcopy or LEEP: Alabama, Michigan, South Carolina and Wyoming. Missouri covers colposcopy and HPV DNA testing, but does not cover LEEP.
All but one state (South Carolina) cover HPV vaccines for young adults in their traditional Medicaid programs, but only 14 of 23 states cover HPV vaccines in their family planning waiver or SPA despite the widespread coverage for pap screening. States must cover the vaccine for ACA expansion groups, as all vaccines recommended by the national Advisory Committee on Immunization Practices (ACIP) are included in the ACA’s preventive services coverage requirement.
Utilization Controls
Only two states noted utilization controls for cancer screening methods. California restricts utilization to women ages 21 through 65 regardless of sexual history. Colorado limits a pap smear and lab to one per year unless additional screens are determined to be medically necessary.
| Table 15: Coverage of Cervical Cancer Services | ||||
| Traditional Medicaid (n=41) |
ACA Medicaid Expansion (n=25) |
Family Planning Waiver/SPA (n=23) |
Covered in Context of Family Planning Visit | |
| HPV Vaccine | 40† | Required | 14: AL, CT, IA, IN, MD, MN, MO, MS, MT, NH, NM, OH, OK, WY | |
| PAP Smear and Lab | 41 | Required | 23: AL, CA, CT, GA, IA, IN, MD, MI, MN, MO, MS, MT, NC, NH, NM, NY, OH, OK, OR, SC, VA, WA, WY | CA, IA*, MO, OR*, VA |
| Follow- up Procedures with Abnormal Pap | ||||
| Colposcopy | 41 | 24 | 14: CA, CT, GA, IA, IN, MD, MN, MO, MS, MT, NH, NM, NY, OH | IA*, MO |
| LEEP | 41 | 24 | 13: CA, CT, GA, IA, IN, MD, MN, MS, MT, NH, NM, NY, OH | |
| HPV DNA Testing | 40ǂ | Required | 18: AL, CA, CT, GA, IA, IN, MD, MI, MN, MO, MS, MT, NH, NM, NY, OH, SC, WY | MO |
| Note: † Not covered in SC. ǂ Not covered in NC. *IA: sometimes considered family planning, depending on context of the visit. OR: Pap covered under state family planning waiver when provided in context of a contraceptive management visit. | ||||
Breast Cancer Services
| Key Finding: Breast Cancer Services |
| Breast cancer screening is a required benefit in ACA Medicaid expansion programs, but it is considered “optional” under traditional Medicaid; however, all states covered this screening under these two programs.
Few states cover breast cancer screening and prevention services through their family planning waiver or SPA. |
The USPSTF recommends three services for women related to detection and prevention of breast cancer: 1) biannual mammograms for women ages 50 to 74 to screen for breast cancer; 2) genetic testing for mutation of the BRCA1 and BRCA2 genes in some women with family members that have breast, ovarian, fallopian tube, or peritoneal cancer; 3) preventive medication for some women with elevated risk of breast cancer. Because these services are all recommended by the USPSTF, they must be covered in Medicaid ACA expansion programs but are not required in traditional or family planning pathways.
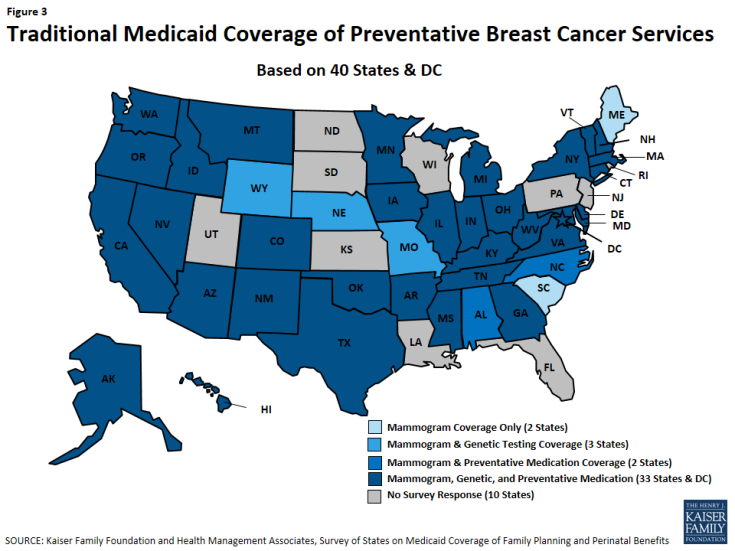
Minnesota, Montana, New Hampshire, and New Mexico cover all three services across all of their eligibility pathways. Thirty-three states and DC cover all of these services under traditional Medicaid (Figure 3). All states cover mammograms, and most cover genetic BRCA screening (37 of 41 states), and breast cancer preventive medication (36 of 41 states) for high-risk women under their traditional Medicaid program (Table 17). Maine and South Carolina do not cover BRCA Screening/Counseling nor breast cancer preventive medication in any of the pathways where coverage for these services is optional.
Coverage of breast cancer screening and prevention under state family planning waivers or SPAs is much less common, with seven of 23 of these programs covering mammograms (Maryland, Minnesota, Montana, New Hampshire, and New Mexico, Ohio, South Carolina) and six states covering BRCA screening and counseling (Minnesota, Montana, New Hampshire, and New Mexico Maryland, Iowa) or preventive breast cancer medication (Minnesota, Montana, New Hampshire, and New Mexico, Ohio, Iowa).
Utilization Controls
No states noted utilization controls for mammograms. Five states have utilization controls applied to BRCA screening and counseling. Michigan, Texas, Vermont and Washington require prior authorization and in Nevada, genetic counseling must precede the testing. Michigan requires prior authorization for preventive medications. Appendix Table A9 provides detail for state responses on breast cancer services.
| Table 17: Coverage for Breast Cancer Screening and Prevention | |||
| Traditional Medicaid (n=41) |
ACA Medicaid Expansion (n=25) |
Family Planning Waiver/SPA (n=23) |
|
| Mammogram | 41 | Required | 7 |
| Genetic (BRCA) Screening and Counseling for High-Risk Women | 37 | Required | 6 |
| Breast Cancer Preventive Medication for High-Risk Women | 36 | Required | 6 |
Managed Care and Family Planning Services
| Key Finding: Managed Care and Family Planning Services |
| Most of the responding states have capitated contracts that include family planning in the capitation rate. Just over one-third of these states explicitly address potential utilization controls on family planning services in the contracts. |
Managed care is now the predominant delivery system for Medicaid in most states. Over three in four women of reproductive age covered by Medicaid are enrolled in managed care arrangements.1
For MCO enrollees, ensuring that federal family planning requirements are met can present special challenges. For example, while MCOs typically limit beneficiaries to a contracted network of providers, in the case of family planning this is not allowed under the federal “freedom of choice” policy. Beneficiaries are entitled to see any Medicaid provider for family planning care, but may not be aware of this right. Some providers have also reported difficulty receiving reimbursement if they are not part of an MCO network. In capitated arrangements, it can also be difficult to know whether the state is obtaining the higher 90% matching rate applicable to family planning services. This survey included questions that explore the role of capitated MCOs in providing family planning services to women enrolled in Medicaid.
Of the 41 states responding to this survey, 31 reported contracting with capitated MCOs and 29 of these states indicated that family planning supplies and services are always included within the MCO capitation rate (Table 18). Two additional states, New York and Texas, indicated that some or all family planning services are carved-out of MCO contracts only but for MCOs claiming a “conscience” or religious exemption from the requirement to provide family planning services.
Twenty-five of the 31 responding states with MCOs reported that they claimed the higher 90 percent federal matching rate (“FMAP”) for family planning services provided through the MCO while five states2 indicated that they did not.
| Table 18: State Reporting on Capitated MCO Contracts | ||
| Yes | No | |
| State has capitated MCO contracts? (N=41) | 31 | 10 |
| State include family planning within capitation rates? (N=31) |
29 – Always |
|
| State claims 90% FMAP for family planning services provided through an MCO? (N=31) | 25 | 6* |
| MCO states contract with MCO with religious exemption? (N= 31) | 4 | 27 |
| MCO contract addresses family planning utilization controls? (N=31) | 11 | 20 |
| * South Carolina was in the process of claiming 90% FMAP | ||
In addition to New York and Texas, only two other states (California and Oregon) reported having contracts with MCOs having a conscience or religious exemption from the requirement to provide family planning services. Only California reported having a referral process for enrollees in these plans, requiring the MCO to arrange for the timely referral and coordination of family planning services and to demonstrate the ability to arrange, coordinate and ensure provision of services through referrals at no additional expense to the state. The MCO is also required to identify these services in its Member Services Guide.
| Key Finding: Faith-Based Plans |
| California and New York, states with the most beneficiaries, also contract with faith-based plans that oppose some forms of contraception. While California reported that they have a process in place for referral for family planning services for the beneficiaries in these plans, New York did not report a referral practice. |
States were also asked whether their contracts with MCOs explicitly address how MCOs can use prior authorization, step therapy or other medical management controls for contraceptives. Eleven of the 31 of the responding states with MCO contracts answered “Yes.” Of these, three states (Illinois, Massachusetts and Texas) indicated that their MCO contracts prohibit prior authorization requirements for contraceptives and Arizona reported that MCOs are “not allowed to create barriers to contraceptive utilization.” Conversely, Maryland indicated that prior authorization and quantity limitations are permitted and Delaware reported that MCOs are required to follow the state’s preferred drug list, which includes contraceptive agents. Three states (Illinois, Maryland and New Mexico) said that MCOs are required to cover all FDA-approved contraceptives. Appendix Table A10 provides additional detail for state managed care policies.
Conclusion
Conclusion
The analysis of state responses to this survey found that overall most states cover a broad range of prescription contraceptive methods in their full scope traditional Medicaid program, their full scope ACA Medicaid expansion, and the family planning expansion programs. Thirty-six of 41 surveyed states report that they cover all prescription contraceptives for women through their full scope programs. While states are not required to cover all methods under all pathways, most do. However, there is more variation between and within states for coverage of over-the-counter contraceptives, including condoms and Plan B emergency contraception pills. In some cases, when states provide coverage, it is only with a prescription which can limit access to these safe and effective methods.
Under traditional Medicaid, many screening and preventive services that are considered family planning “related services”, such as screening for partner violence and preventive therapies for breast cancer for women at elevated risk are optional. While coverage is provided for many women who qualify for the full scope pathways (traditional Medicaid or ACA expansion), coverage is less consistent for women who qualify for family planning expansions programs (SPA or Waiver). These are services that often allow providers to address underlying health and personal issues that affect sexual and reproductive health for women and men; limited reimbursement options mean that many will not get these services because providers do not have a way to charge for this care. This is not an issue that is unique to family planning and has long plagued the health care system for a broad array of preventive health care issues.1
The survey also finds that several states are actively working to promote access to LARC contraception methods. Most states provided coverage for the different types of IUDs that are available under all of their pathways and without utilization limits such as prior authorization or step therapy. Use of LARCs has increased significantly in recent years and the provider community has been encouraging greater use. Most recently, the American College of Obstetricians and Gynecologists endorsed broader provision of LARC methods postpartum.2
However, there are a number of financial and administrative disincentives that have made it challenging for clinicians to facilitate access to LARCs for women who want them. Policies including the integration of the cost of postpartum LARC insertion with the global maternity fee, low reimbursement levels, and administrative requirements can make it difficult for providers to retain a stock of IUDs. Given that Medicaid finances roughly half all birth in the US, state Medicaid policy on reimbursement of post-partum LARCs has the potential to broaden access to these effective methods for women who desire them. An April 2016 bulletin from CMS outlines a number of options for states to facilitate the use of post-partum LARC, including: increasing reimbursement, unbundling post-partum LARC insertion from the typical maternity care global fee, reducing administrative and logistical barriers to stocking IUDs, and providing them the same day a patient visits her provider.3 The survey finds that a number of states are providing payment separate from the maternity fee for postpartum LARCs, a change that has likely taken place in recent years as documented by other research.4
Medicaid enrollment has risen significantly since the ACA’s passage and could further increase if all states take up the ACA option to expand the program to all individuals living below 138% of the poverty level. Short of that, some of the states that have chosen not to expand full scope Medicaid have opted to establish family planning programs through Medicaid waivers or SPAs. These programs offer access to some family planning services, particularly contraceptives, but often not to the same degree as full scope Medicaid. Access to the full range of contraceptive methods as well as related family planning services has become a standard of comprehensive health care for women and men in their reproductive years.5 As enrollment in the Medicaid program continues to grow as a result of the ACA, coverage of family planning services under Medicaid will continue to be a significant force in shaping access to sexual and reproductive health services for low-income women and men in years to come.
Appendices
Appendix A: State-Level Survey Results (PDF)
Appendix B: Medicaid Coverage Requirements for Family Planning Services, by Eligibility Pathway (PDF)
Appendix C: Methodology (PDF)
Appendix D: Survey Questionnaire – Family Planning Section (PDF)
Endnotes
Executive Summary
Food and Drug Administration (FDA), Approved methods of contraception for women.
Gavin, L., et al. Providing Quality Family Planning Services, MMWR, April 25, 2014.
Introduction
Kaiser Family Foundation; Status of State Action on the Medicaid Expansion Decision; As of January 12, 2016; Since July 1, 2015 Alaska implemented a Medicaid expansion (9/1/2015) as did Montana (1/1/2016) and Louisiana’s (7/1/2016).
Guttmacher Institute, Medicaid Family Planning Eligibility Expansions, State Policies in Brief, as of October 1, 2015.
Gold, RB. “Doing More for Less: Study Says State Medicaid Family Planning Expansions Are Cost-Effective.” Guttmacher Report on Public Policy, March 2004.
Kaiser Family Foundation. Status of State Action on the Medicaid Expansion Decision.
Kaiser Family Foundation. Women and Health Insurance, November 2013
Reversible Contraception
Cooper, C., et al. Interpregnancy Intervals in the United States: Data From the Birth Certificate and the National Survey of Family Growth, National Vital Statistics Reports, April 16, 2015.
All Patient Refined Diagnosis Related Group. Currently three major versions of the DRG in use include: basic DRGs, All Patient DRGs, and All Patient Refined DRGs. DRGs, used by Medicare, measure the typical resource use of an inpatient stay. AP-DRGs are similar to DRGs, but also include a more detailed DRG breakdown for non-Medicare patients such as newborns and children. The APR-DRG structure is similar to AP-DRG, but also measures severity of illness and risk of mortality in addition to resource utilization. See: Jason Shafrin; What is the Difference Between DRGs, AP-DRGs and APR-DRGs; Healthcare Economist; June 2012; accessed at: http://healthcare-economist.com/2012/06/19/what-is-the-difference-between-drgs-ap-drgs-and-apr-drgs/
This survey was conducted prior to California’s implementation of policy requiring Medicaid plans to cover 12 month supply of oral contraceptives.
Plan B, progestin, brand names include Plan B One Step, Next Choice One Dose, My Way and Take Action.
In Indiana, contraceptive drugs and supplies may be administered, dispensed, prescribed, or ordered. However, for a pharmacy provider to be reimbursed for over-the counter external contraceptive supplies, a Medicaid practitioner with prescriptive authority must prescribe them. IHCP Bulletin BT201301; January 8, 2013 accessed at: http://provider.indianamedicaid.com/ihcp/Bulletins/BT201301.pdf.
Sterilization Procedures
42 CFR §441.250 through 441.259
Managed Care and Family Planning Services
Kaiser Family Foundation, Medicaid’s Role for Family Planning, July 2015.
One of the five states reporting that it does not claim the 90 percent FMAP (Idaho) contracts with only one MCO to provide services in the state’s Medicare Medicaid Coordinated Plan (MMCP), a type of Fully Integrated Dual Eligible Special Needs Plan that enrolls individuals over the age of 21 that are eligible for both Medicare and Medicaid. For a description of the MMCP, see: http://healthandwelfare.idaho.gov/Medical/Medicaid/MedicaidParticipants/MedicareMedicaidCoordinatedPlan/tabid/2538/Default.aspx.
Conclusion
Yarnall, et al. Primary Care: Is there Enough Time for Prevention?, AJPH, 2002.
ACOG Committee on Obstetric Practice (2016). Immediate Postpartum Long Acting Reversible Contraception.
Centers for Medicare and Medicaid Services (2016). State Medicaid Payment Approaches to Improve Access to Long-Acting Reversible Contraception.
Moniz, M., et al. Medicaid Administrator Experiences with the Implementation of Immediate Postpartum Long-Acting Reversible Contraception, Women’s Health Issues, May-June 2016.
Gavin, L., et al. Providing Quality Family Planning Services, MMWR, April 25, 2014.